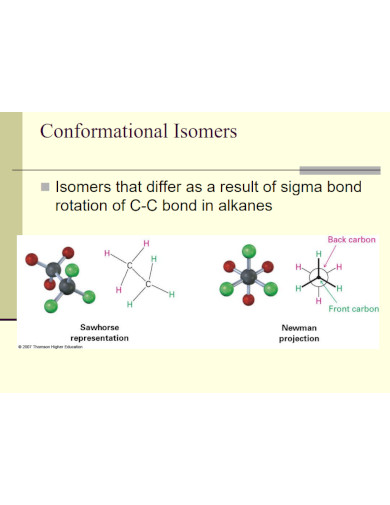
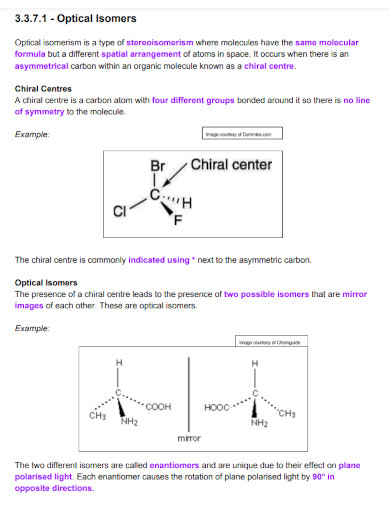
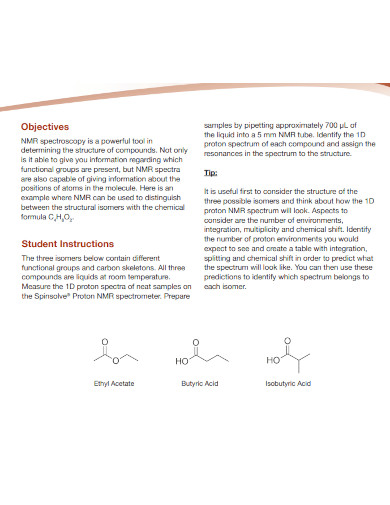
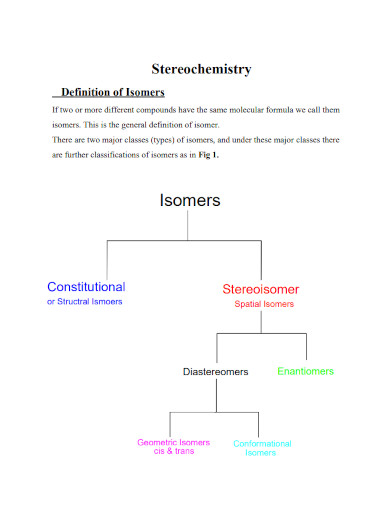
What defines isomers in chemistry?
Compounds with the same molecular formula but different structures
Compounds with different molecular formulas and structures
Compounds that have identical physical and chemical properties
Compounds with the same molecular weight and different atoms