The comprehensive guide on Erbium, a pivotal element in the world of science and technology. This article offers an insightful exploration of Erbium, detailing its definition, intrinsic properties, and the multitude of its applications ranging from telecommunications to medical equipment. Through illustrative examples, you’ll discover why Erbium is not just another element but a cornerstone in advancing modern innovations. Enhance your understanding of how Erbium’s unique characteristics and compounds contribute to its critical role across various industries.
What is Erbium?
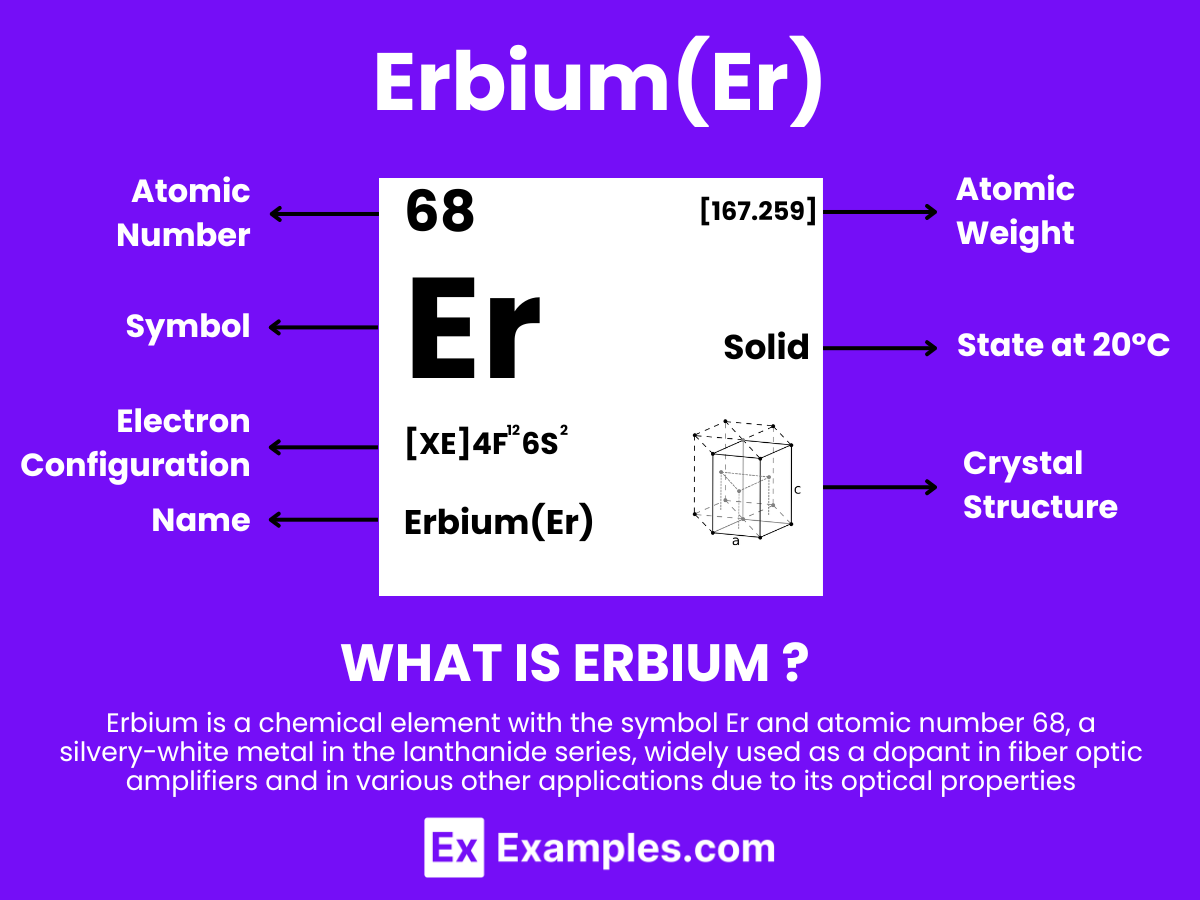
Erbium is a silvery-white metallic element belonging to the lanthanide series of the periodic table, with the atomic number 68. Known for its pink-colored ions, erbium finds extensive use in optical fiber amplifiers, lasers, and in the coloring of glasses and ceramics. Its natural occurrence is mainly found in minerals like xenotime and euxenite, often extracted through complex chemical processes. Erbium’s unique properties, such as its ability to absorb infrared light, make it an indispensable component in modern telecommunications, offering the potential for significant advancements in technology and materials science.
Erbium Formula
- Formula: Er
- Composition: Consists of a single erbium atom.
- Bond Type: In its elemental form, erbium does not form bonds as it is a pure element. However, erbium can participate in covalent or ionic bonding when reacting with other elements, leveraging its position within the lanthanide series of the periodic table.
- Molecular Structure: As a pure element, erbium does not present a molecular structure in the traditional sense of compounds. Due to its metallic nature, in bulk material, erbium is expected to exhibit a metallic state with a crystalline structure typical of lanthanide metals, often hexagonal close-packed (hcp).
- Electron Sharing: In compounds, erbium is expected to share electrons covalently or engage in ionic electron transfer with other elements. This behavior is typical for lanthanides, which can form a variety of compounds with different elements, displaying both ionic and covalent bonding characteristics.
- Significance: Erbium’s significance lies in its various applications driven by its unique properties, such as being a component in optical fibers and lasers. Its ability to absorb certain wavelengths efficiently makes it valuable in technology and materials science.
- Role in Chemistry: The role of erbium in chemistry extends beyond theoretical interest, encompassing practical applications in materials science, technology, and medicine. Its compounds are used in a variety of contexts, from improving the performance of optical fibers to serving as dopants in laser materials, highlighting the practical implications of erbium’s chemical behavior and interactions.
Atomic Structure of Erbium
Erbium, in contrast to hydrogen, is a metallic element with characteristics that reflect its status as a member of the lanthanide series of the periodic table. Its properties include a metallic luster, malleability, and conductivity, which significantly diverge from the gaseous nature and simple molecular formation of hydrogen. Erbium’s behavior at the atomic and molecular levels is defined by its electronic structure and its role in various chemical and physical processes.
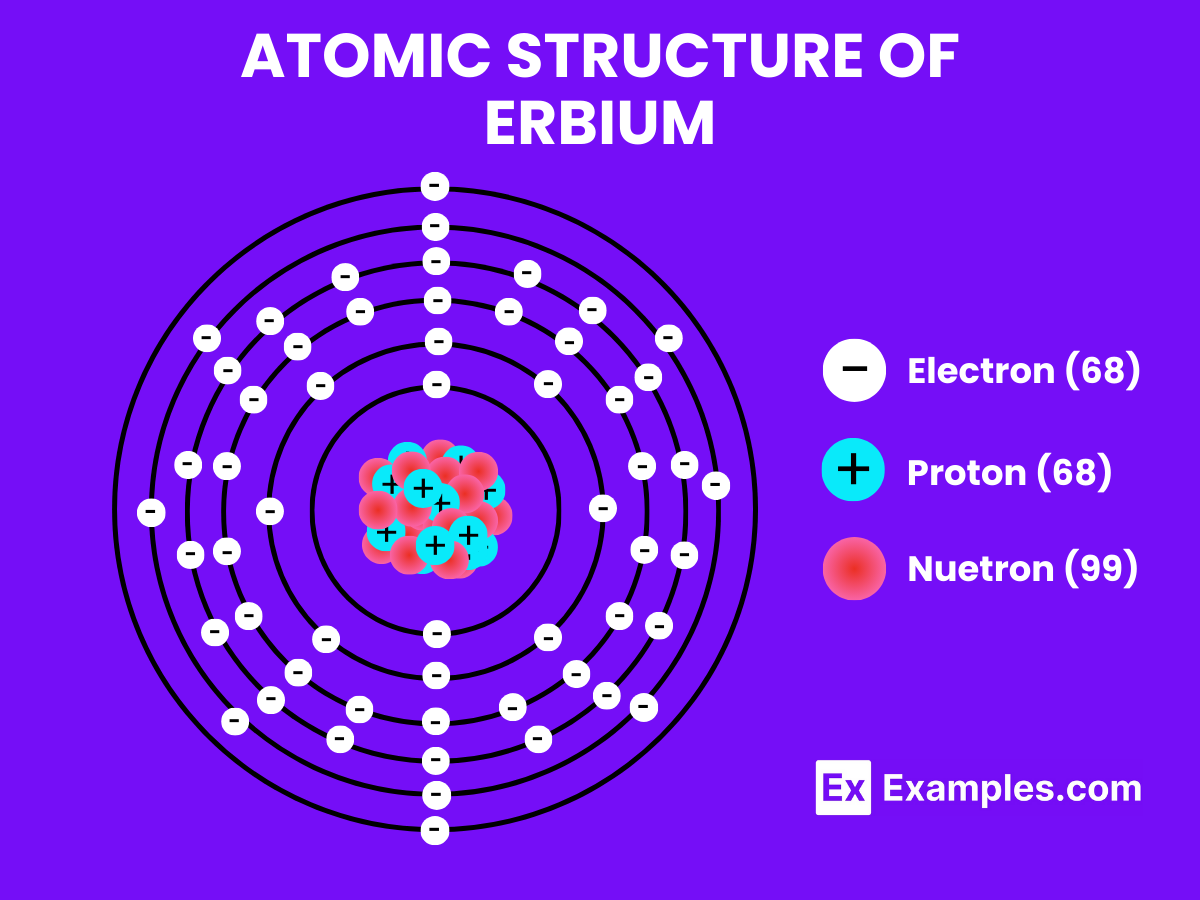
Atomic Level: Each erbium atom (Er) contains 68 protons in its nucleus and is expected to have 68 electrons orbiting around it. The electron configuration of erbium is predicted to be [Xe] 4f¹² 6s², indicating a complex electron configuration with potential for various oxidation states, similar to other elements in the lanthanide series. This suggests a certain level of chemical reactivity, allowing erbium to form compounds with other elements. The partially filled 4f electron shell of erbium contributes to its unique optical and magnetic properties.
Molecular Formation: Unlike hydrogen, which forms simple diatomic molecules (H₂) through covalent bonding, erbium would not form molecules in a similar manner due to its metallic nature. In bulk form, erbium is expected to exhibit a metallic lattice structure typical of metals. This structure involves metallic bonding, where electrons are delocalized over many erbium atoms, differing fundamentally from the discrete electron sharing seen in hydrogen’s covalent bonds. The crystalline structure of solid erbium is usually hexagonal close-packed (hcp), which is common among rare earth metals.
Properties of Erbium
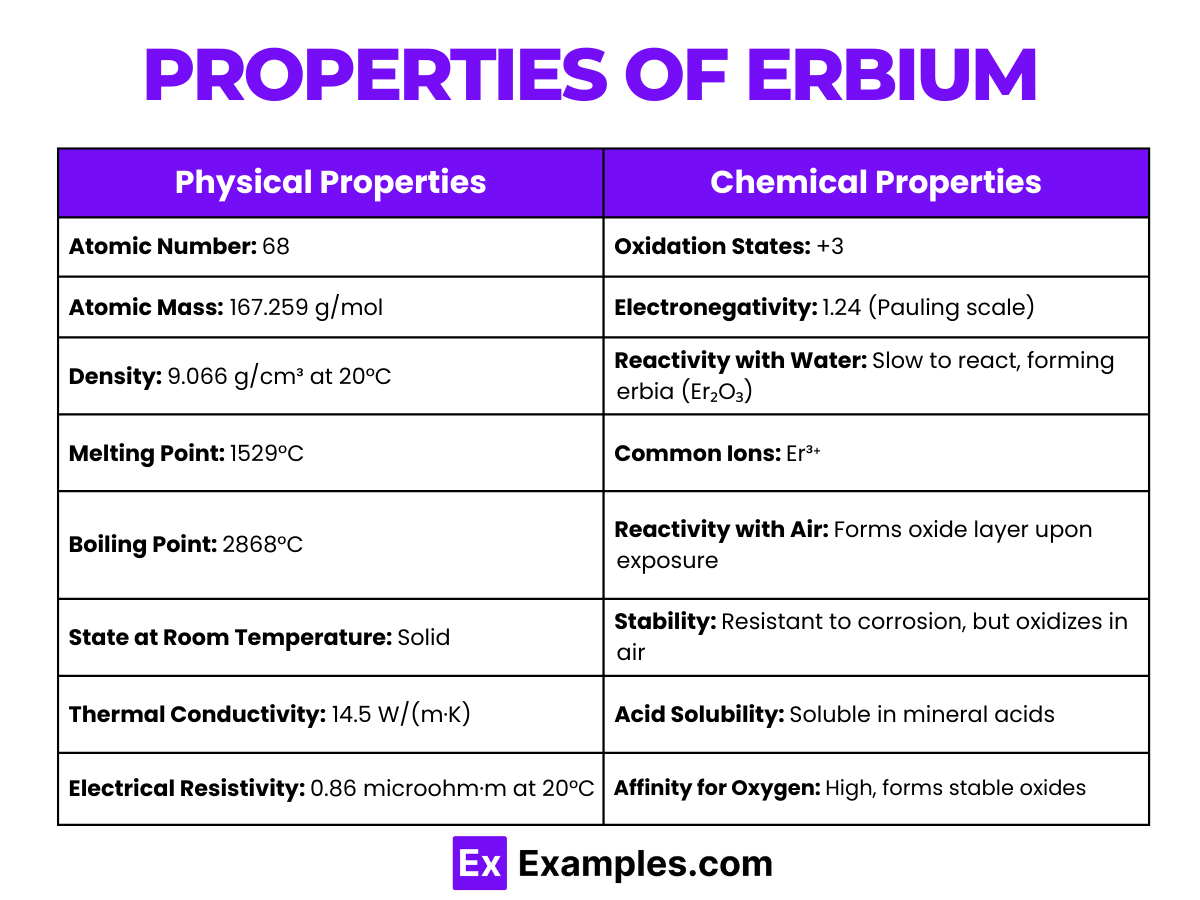
| Physical Properties | Chemical Properties |
|---|---|
| Atomic Number: 68 | Oxidation States: +3 |
| Atomic Mass: 167.259 g/mol | Electronegativity: 1.24 (Pauling scale) |
| Density: 9.066 g/cm³ at 20°C | Reactivity with Water: Slow to react, forming erbia (Er₂O₃) |
| Melting Point: 1529°C | Common Ions: Er³⁺ |
| Boiling Point: 2868°C | Reactivity with Air: Forms oxide layer upon exposure |
| State at Room Temperature: Solid | Stability: Resistant to corrosion, but oxidizes in air |
| Thermal Conductivity: 14.5 W/(m·K) | Acid Solubility: Soluble in mineral acids |
| Electrical Resistivity: 0.86 microohm·m at 20°C | Affinity for Oxygen: High, forms stable oxides |
Physical Properties of Erbium
| Property | Value |
|---|---|
| Appearance | Silvery-white, soft metal |
| Atomic Mass | 167.259 g/mol |
| Melting Point | 1529 °C |
| Boiling Point | 2868 °C |
| Density | 9.066 g/cm³ at 20 °C |
| State at 20 °C | Solid |
| Electrical Conductivity | Good conductor |
| Thermal Conductivity | 14.5 W/(m·K) at 25 °C |
| Magnetic Ordering | Paramagnetic |
Chemical Properties of Erbium
Erbium, like other lanthanides, has interesting chemical properties due to its electron configuration and its position in the periodic table. It tends to form compounds in the +3 oxidation state, which is common among the rare earth elements.
Examples of Chemical Properties and Equations:
- Reaction with Water:
- Erbium reacts with water to form erbium(III) hydroxide and hydrogen gas.
- Equation:2Er+6H₂O→2Er(OH)₃+3H₂
- Reaction with Oxygen:
- When exposed to air, erbium forms an oxide layer of erbium(III) oxide.
- Equation:
- Reaction with Acids:
- Erbium dissolves in dilute hydrochloric acid to form erbium(III) chloride and hydrogen gas.
- Equation: Er+6HCl→ErCl₃+3H₂
- Formation of Alloys:
- Erbium readily forms alloys with other metals, enhancing their properties
Thermodynamic Properties of Erbium
| Property | Value |
|---|---|
| Standard Molar Entropy (S°298) | 62.9 J/(mol·K) |
| Heat of Fusion | 19.90 kJ/mol |
| Heat of Vaporization | 280 kJ/mol |
| Standard Enthalpy of Formation (ΔHf°298) | -165.4 kJ/mol for Er_2O_3 |
Material Properties of Erbium
| Property | Value |
|---|---|
| Crystal Structure | Hexagonal Close-Packed (hcp) |
| Hardness | Soft metal, Mohs hardness ~2.5 |
| Elastic Modulus | 69.9 GPa |
| Poisson’s Ratio | 0.237 |
| Thermal Expansion Coefficient | 12.2 µm/(m·K) at 25 °C |
Electromagnetic Properties of Erbium
| Property | Description |
|---|---|
| Magnetic Ordering | Paramagnetic at room temperature |
| Electrical Resistivity | 0.86 microohm·m at 20°C |
| Thermal Conductivity | 14.5 W/(m·K) |
| Optical Properties | High absorption in visible, infrared, and near-infrared; used in optical fibers |
| Magnetic Susceptibility | Strongly paramagnetic over a wide temperature range |
| Laser Activity | Erbium-doped fibers used in lasers for their efficient emission in the 1.55 µm range |
Nuclear Properties of Erbium
| Property | Description |
|---|---|
| Natural Isotopes | Er-162, Er-164, Er-166, Er-167, Er-168, Er-170 |
| Most Stable Isotope | Er-166 (stable) |
| Neutron Cross Section | Moderate, varies by isotope |
| Neutron Absorption | Used in nuclear technology for its neutron absorption capabilities |
| Isotopic Abundance in Nature | Er-166 is the most abundant isotope |
| Radioactive Isotopes | Several, including Er-169 used in medicine for its gamma radiation |
Preparation of Erbium
Erbium is typically prepared through complex extraction and reduction processes from minerals like xenotime and monazite, which contain a mixture of rare earth elements. The preparation involves several steps:
- Extraction: The initial step involves extracting the mixed rare earth elements from the mineral ore. This is commonly achieved through an ion exchange process or solvent extraction techniques, separating the rare earth elements from the other components of the ore.
- Fractional Crystallization or Ion Exchange: After extraction, the rare earth elements are separated from each other. This can be accomplished through fractional crystallization of their double sulfate salts or by using ion exchange techniques, leveraging the slight differences in their chemical properties.
- Reduction: The purified erbium oxide (Er₂O₃) is then reduced to metallic erbium. This reduction is typically done using either electrolysis or a chemical reduction method. In the chemical reduction method, erbium oxide is reacted with a reducing agent, such as lanthanum metal or calcium, in a high-temperature process.
Chemical Compounds of Erbium
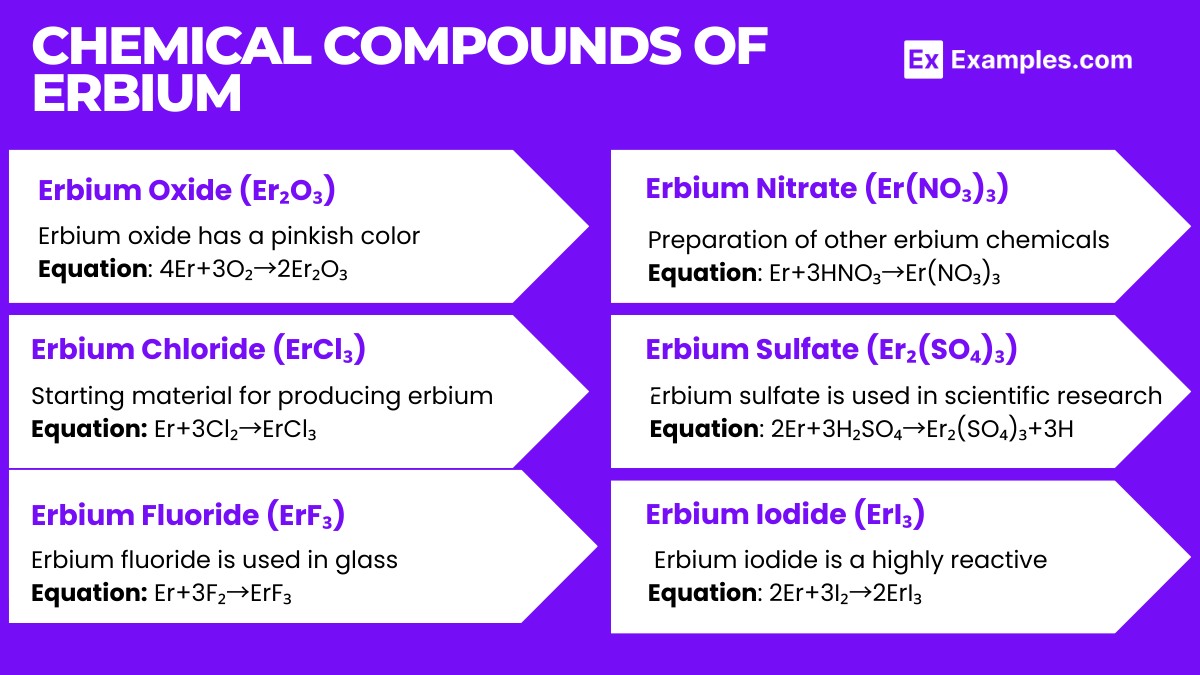
- Erbium Oxide (Er₂O₃):
- Equation: 4Er+3O₂→2Er₂O₃
- Properties: Erbium oxide has a pinkish color and is used in ceramic glazes and as a colorant in glassmaking. It is also used in nuclear reactor control rods.
- Erbium Chloride (ErCl₃):
- Equation: Er+3Cl₂→ErCl₃
- Properties: This compound is utilized in the synthesis of other erbium compounds. It serves as a starting material for producing erbium metal in its pure form.
- Erbium Fluoride (ErF₃):
- Equation: Er+3F₂→ErF₃
- Properties: Erbium fluoride is used in glass, optics, and lasers due to its low phonon energy, which is advantageous for upconversion processes in laser applications.
- Erbium Nitrate (Er(NO₃)₃):
- Equation: Er+3HNO₃→Er(NO₃)₃
- Properties: This compound is used in the preparation of other erbium chemicals and as a catalyst in organic synthesis.
- Erbium Sulfate (Er₂(SO₄)₃):
- Equation: 2Er+3H₂SO₄→Er₂(SO₄)₃+3H
- Properties: Erbium sulfate is used in scientific research and as a dopant in the preparation of various materials
- Erbium Iodide (ErI₃):
- Equation: 2Er+3I₂→2ErI₃
- Properties: Erbium iodide is a highly reactive compound used primarily in the synthesis of other erbium-containing materials.
Isotopes of Erbium
| Isotope | Mass Number | Half-life | Primary Decay Mode |
|---|---|---|---|
| Er-162 | 162 | Stable | – |
| Er-164 | 164 | Stable | – |
| Er-166 | 166 | Stable | – |
| Er-167 | 167 | Stable | – |
| Er-168 | 168 | Stable | – |
| Er-169 | 169 | 9.4 days | Beta decay |
| Er-170 | 170 | Stable | – |
| Er-172 | 172 | Stable | – |
Uses of Erbium
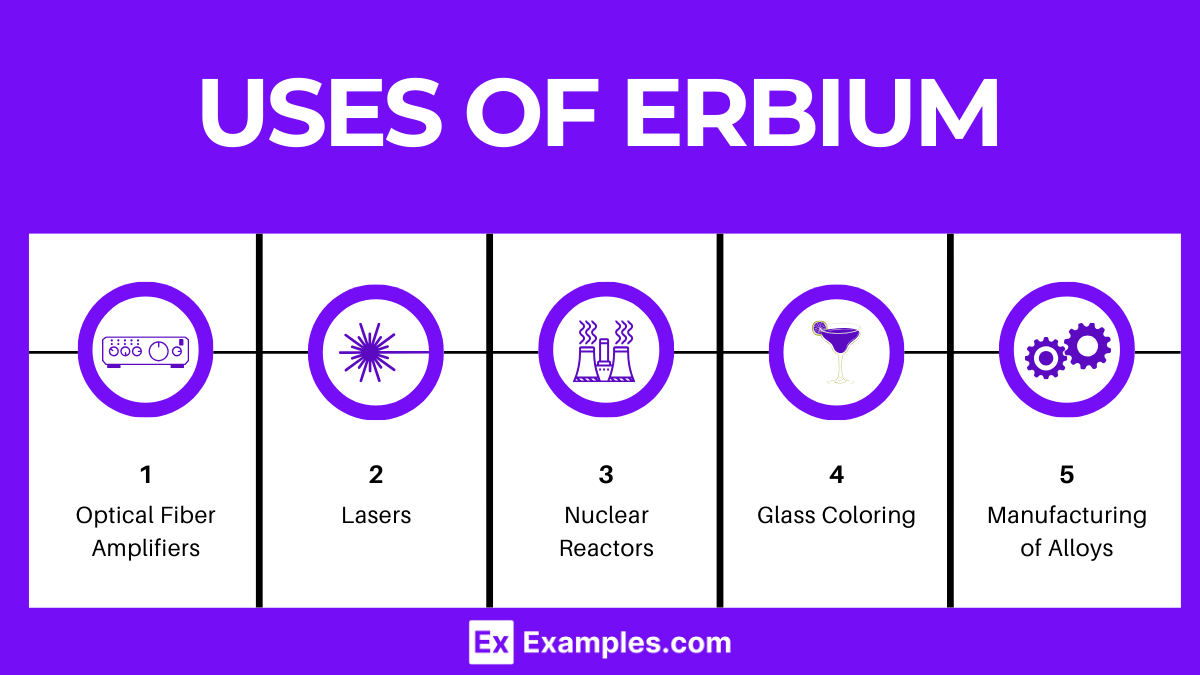
- Optical Fiber Amplifiers: Erbium-doped fiber amplifiers (EDFAs) are widely used in telecommunications to amplify light signals without converting them into electrical signals, enhancing the efficiency and capacity of fiber-optic communication systems.
- Lasers: Erbium-doped lasers are utilized in various fields, including dermatology for skin resurfacing, dentistry for precision cutting, and surgery for minimally invasive procedures.
- Nuclear Reactors: Erbium is used as a neutron absorber in nuclear reactors to control the fission process, thanks to its ability to capture neutrons effectively.
- Glass Coloring: Erbium oxide provides a pink color to glasses and ceramics, used in both artistic and industrial applications to produce tinted materials.
- Manufacturing of Alloys: Erbium is added to vanadium to improve its workability and to other metals to enhance their mechanical properties.
- Photonic Crystals: Utilized in the construction of photonic crystal fibers, erbium can enhance the properties of these materials for use in advanced optical devices.
- Quantum Computing: Erbium ions are researched for their potential use in quantum computing and quantum communications, owing to their unique quantum mechanical properties.
- Medical Imaging: Erbium compounds are explored for use in medical imaging technologies, including magnetic resonance imaging (MRI), to improve the contrast and quality of images.
Production of Erbium
The production of erbium is a detailed process that involves several stages, from mining rare earth minerals to isolating and refining the erbium metal. Erbium is typically extracted from minerals such as xenotime and monazite, which contain a mix of rare earth elements. The key steps in erbium production include:
- Extraction: The first step involves mining the rare earth minerals that contain erbium. These minerals are processed to extract the rare earth elements as a mixed concentrate.
- Separation: The mixed rare earth concentrate undergoes separation processes, such as solvent extraction and ion exchange, to separate erbium from other rare earth elements. This step is crucial due to the similarity in chemical properties among the lanthanides.
- Metallic Reduction: Once erbium is purified to erbium oxide (Er2O3), it is reduced to metallic erbium using reducing agents such as calcium or lanthanum. This process typically occurs at high temperatures in an inert atmosphere to prevent oxidation.
- Refining: The erbium metal is further refined to remove any impurities, ensuring high purity for industrial or commercial use. This may involve additional steps such as vacuum distillation or electrorefining.
- Forming: Finally, the refined erbium metal is formed into various shapes, such as ingots, sheets, or powders, depending on its intended application. This is done through processes such as melting, casting, and milling
Applications of Erbium
Erbium has a variety of applications across different fields, leveraging its unique properties, especially its optical characteristics. Some of the primary applications include:
- Fiber Optics: Erbium-doped fiber amplifiers (EDFAs) are crucial in long-distance fiber optic communication systems. They amplify the signal light in telecommunications and internet cables without the need to convert it back to electrical signals.
- Lasers: Erbium-doped lasers are used in medical applications, such as dermatology and dentistry, for skin resurfacing and tooth enamel ablation. These lasers are preferred for their ability to be absorbed by water and biological tissues, allowing precise cutting with minimal damage.
- Glass Coloring: Erbium oxide is used to impart a pink color to glasses and ceramics, utilized in both decorative and functional applications.
- Nuclear Reactors: Due to its high neutron absorption cross-section, erbium can be used as a neutron poison in nuclear reactors to control the fission process.
- Metallurgy: Erbium can improve the workability and mechanical strength of alloys, particularly those used in aerospace applications.
- Quantum Computing: Erbium’s unique quantum states in certain crystal structures are being researched for potential use in quantum computing and information storage technologies.
This article meticulously explores erbium’s journey from production to its pivotal applications, unveiling the element’s electromagnetic and nuclear properties. Erbium’s role in enhancing telecommunications and medical technologies exemplifies its importance. By delving into its unique characteristics, the article illuminates erbium’s contribution to advancing modern technological landscapes, underscoring the element’s indispensable value in science and industry.