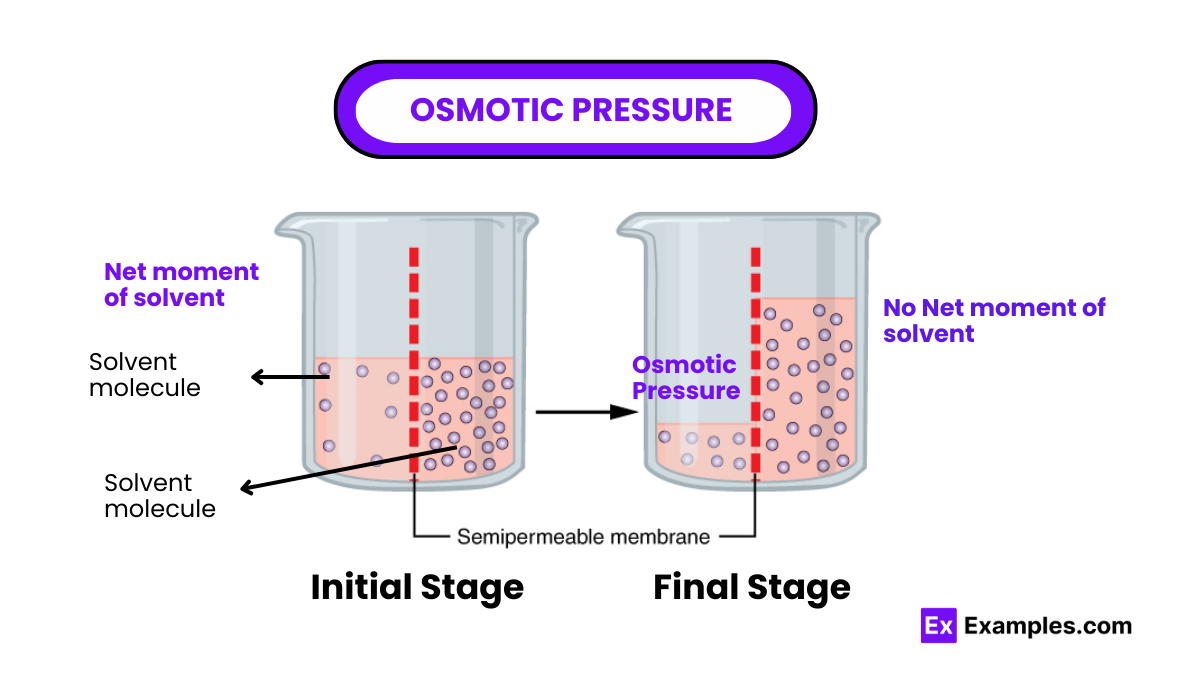
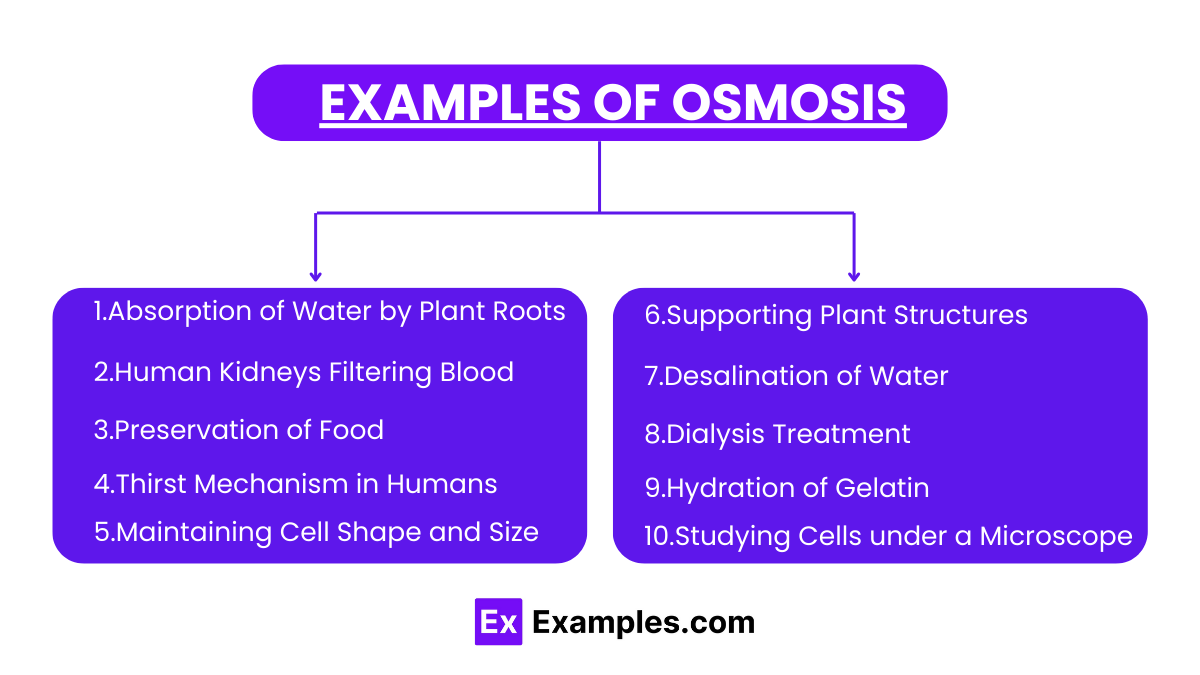
What is the primary definition of osmosis?
The movement of solutes from high to low concentration
he movement of water from high to low concentration through a semipermeable membrane
The movement of water from low to high concentration through a permeable membrane
The diffusion of gases across a membrane