What is an ionic bond?
A bond formed by sharing electrons
A bond formed by transferring electrons
A bond formed by sharing protons
A bond formed by transferring protons
Everything around us is a melting pot of various atomic-level chemical bonds that create a working system. These bonds can be electrically or neutrally charged based on the characteristics taken on by the atom.
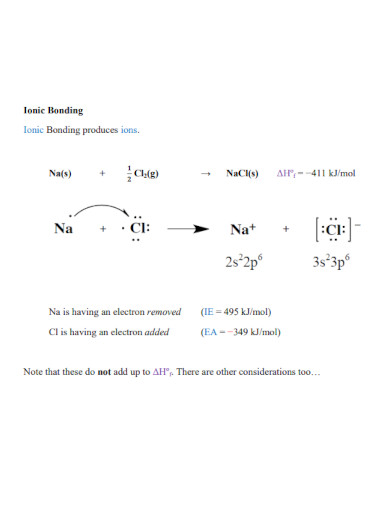
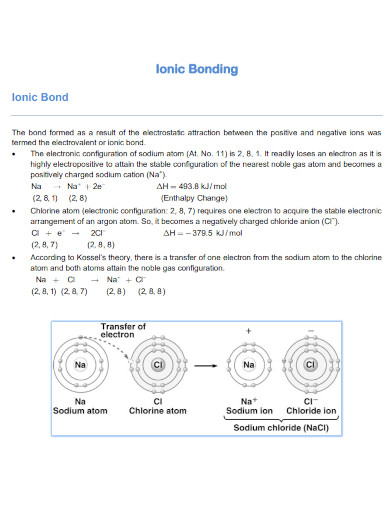
Ionic bonds are the chemical connection between two atoms. An ionic bond is formed when a specific atom transfers its electrons to another atom, forming an ionic bond. This in turn will create a product called an ionic compound that has plenty of identifiable traits.
There are two main types of compounds that are created when chemists form said compounds through the chemical mixing of atoms. These two types of compounds are ionic and covalent compounds. The type of bond the compound uses dictates what type of compound is made. If you are still confused about the concept of the ionic compound or bond, then you are free to read and peruse any of the ionic bond examples, samples, and pdfs on the list above.
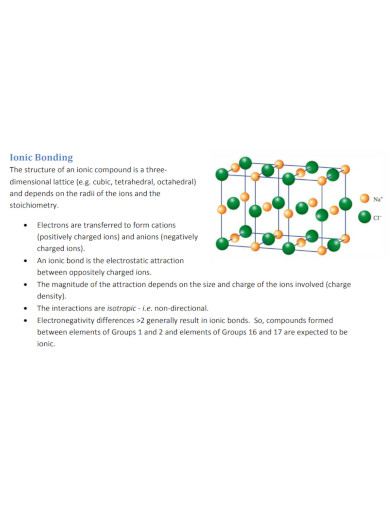
Begin by checking if the compound is exhibiting any of the shared properties of an ionic compound. Most ionic compounds are crystalline in appearance and have high melting and freezing points. These compounds are soluble in water and will often release an electrical charge when mixed with water.
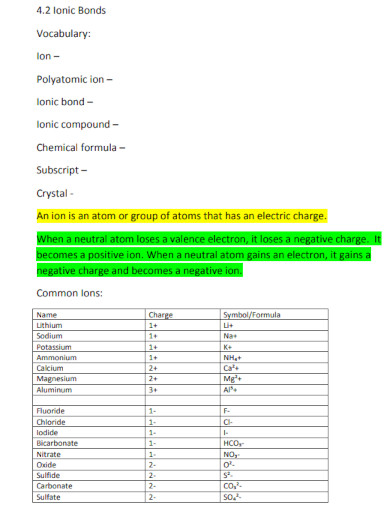
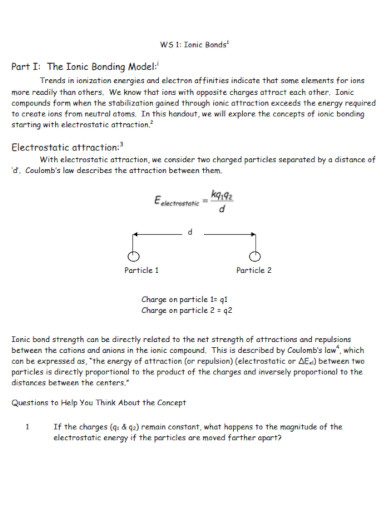
All ionic compounds are formed and share an ionic bond. This means that the atoms composing the compound will be a combination of a negatively charged ion, an anion, and a positively charged ion called a cation.
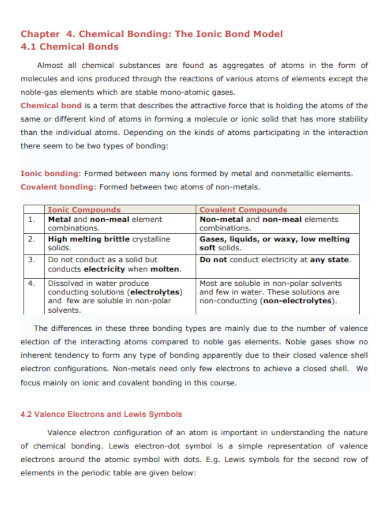
The main difference between ionic and covalent compounds is the electrical charges these two compounds hold. Water is an example of a covalent compound and is neutrally charged without the interference of outside forces. While salt, an ionic compound releases a small electrical charge when it is mixed with water due to its ionic composition.
There are two types of chemical bonds in chemistry these two types come in the form of ionic and covalent bonds. An ionic bond is a chemical bond between two or more atoms that transfer energy from one to the other. The resulting atoms and compounds in the ionic chemical bond will be electrically charged. A covalent bond is a chemical bond where two or more atoms share their energy with each other. The resulting atoms and compounds of the covalent bond are not electrically charged and are considered neutrally charged. These are the main differences between both ionic and covalent bonds.
There are plenty of ionic compounds we can find in our everyday lives, this is because most ionic compounds are soluble in water. NaCl or sodium chloride, also known as table salt, is a commonly used ionic compound. This is because Na or sodium atoms transfer their electrons to the Cl or chlorine atom to form NaCl. Another example of an ionic compound is NaHCO3 or sodium bicarbonate also known as baking soda. All ionic compounds are a product of the combination of a cation and an anion.
The understanding of ionic compounds allows chemists to predict and ensure the synthesis or combination of both atoms results in an ionic compound. Not only will this allow chemists to predict the creation of an ionic compound it will also let the chemist edit or take advantage of the ionic properties or characteristics of the compound to create the desired product or compound.
Ionic bonds are chemical bonds that indicate the successful transfer of one energy to another forming ionic compounds. These ionic compounds have multitudes of uses due to their property of being very miscible and soluble in water. In conclusion, ionic bonds make up a portion of the things we see and use in our everyday lives.
Everything around us is a melting pot of various atomic-level chemical bonds that create a working system. These bonds can be electrically or neutrally charged based on the characteristics taken on by the atom.

tgc.ac.in
Details
File Format
Size: 730 KB

edu.rsc.org
Details
File Format
Size: 43 KB
citycollegekolkata.org
Details
File Format
Size: 160 KB
chem.latech.edu
Details
File Format
Size: 554 KB
pleasantvalleysd.org
Details
File Format
Size: 144 KB
uu.edu
Details
File Format
Size: 156 KB

publish.csiro.au
Details
File Format
Size: 3 MB

ruesd.net
Details
File Format
Size: 59 KB

profiles.uonbi.ac.ke
Details
File Format
Size: 72 KB

whs.rocklinusd.org
Details
File Format
Size: 174 KB

saultschools.org
Details
File Format
Size: 438 KB
roche.camden.rutgers.edu
Details
File Format
Size: 721 KB

cronodon.com
Details
File Format
Size: 1015 KB

hoodriver.or.us
Details
File Format
Size: 273 KB
images.topperlearning.com
Details
File Format
Size: 500 KB
sydney.edu.au
Details
File Format
Size: 166 KB

webassign.net
Details
File Format
Size: 334 KB

westfield.ma.edu
Details
File Format
Size: 8 KB

oasisacademyisleofsheppey.org
Details
File Format
Size: 933 KB
laney.edu
Details
File Format
Size: 91 KB

surendranathcollege.ac.in
Details
File Format
Size: 708 KB

rjuhsd.us
Details
File Format
Size: 31 KB
chemrevise.files.wordpress.com
Details
File Format
Size: 529 KB
cbsd.org
Details
File Format
Size: 468 KB
cityofderbyacademy.org
Details
File Format
Size: 460 KB
Ionic bonds are the chemical connection between two atoms. An ionic bond is formed when a specific atom transfers its electrons to another atom, forming an ionic bond. This in turn will create a product called an ionic compound that has plenty of identifiable traits.
There are two main types of compounds that are created when chemists form said compounds through the chemical mixing of atoms. These two types of compounds are ionic and covalent compounds. The type of bond the compound uses dictates what type of compound is made. If you are still confused about the concept of the ionic compound or bond, then you are free to read and peruse any of the ionic bond examples, samples, and pdfs on the list above.
Begin by checking if the compound is exhibiting any of the shared properties of an ionic compound. Most ionic compounds are crystalline in appearance and have high melting and freezing points. These compounds are soluble in water and will often release an electrical charge when mixed with water.
All ionic compounds are formed and share an ionic bond. This means that the atoms composing the compound will be a combination of a negatively charged ion, an anion, and a positively charged ion called a cation.
The main difference between ionic and covalent compounds is the electrical charges these two compounds hold. Water is an example of a covalent compound and is neutrally charged without the interference of outside forces. While salt, an ionic compound releases a small electrical charge when it is mixed with water due to its ionic composition.
There are two types of chemical bonds in chemistry these two types come in the form of ionic and covalent bonds. An ionic bond is a chemical bond between two or more atoms that transfer energy from one to the other. The resulting atoms and compounds in the ionic chemical bond will be electrically charged. A covalent bond is a chemical bond where two or more atoms share their energy with each other. The resulting atoms and compounds of the covalent bond are not electrically charged and are considered neutrally charged. These are the main differences between both ionic and covalent bonds.
There are plenty of ionic compounds we can find in our everyday lives, this is because most ionic compounds are soluble in water. NaCl or sodium chloride, also known as table salt, is a commonly used ionic compound. This is because Na or sodium atoms transfer their electrons to the Cl or chlorine atom to form NaCl. Another example of an ionic compound is NaHCO3 or sodium bicarbonate also known as baking soda. All ionic compounds are a product of the combination of a cation and an anion.
The understanding of ionic compounds allows chemists to predict and ensure the synthesis or combination of both atoms results in an ionic compound. Not only will this allow chemists to predict the creation of an ionic compound it will also let the chemist edit or take advantage of the ionic properties or characteristics of the compound to create the desired product or compound.
Ionic bonds are chemical bonds that indicate the successful transfer of one energy to another forming ionic compounds. These ionic compounds have multitudes of uses due to their property of being very miscible and soluble in water. In conclusion, ionic bonds make up a portion of the things we see and use in our everyday lives.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is an ionic bond?
A bond formed by sharing electrons
A bond formed by transferring electrons
A bond formed by sharing protons
A bond formed by transferring protons
Which of the following pairs of elements is most likely to form an ionic bond?
H and O
Na and Cl
C and H
O and O
Which of the following compounds is an example of an ionic compound?
H2O
CO2
KBr
CH4
What type of structure do ionic compounds typically form?
Molecular
Metallic
Covalent network
Crystal lattice
Which of the following properties is characteristic of ionic compounds?
High melting and boiling points
High electrical conductivity in solid state
Low solubility in water
Low melting and boiling points
What happens to the electrons in the formation of an ionic bond between sodium (Na) and fluorine (F)?
Sodium and fluorine share electrons equally
Sodium gains electrons from fluorine
Sodium loses electrons to fluorine
Fluorine loses electrons to sodium
Which element is likely to form an ionic bond with chlorine?
Helium
Nitrogen
Potassium
Sulfur
Which of the following statements is true about ionic compounds in aqueous solutions?
They do not conduct electricity
They conduct electricity
They precipitate out of the solution
They form covalent bonds
Which property of ionic compounds allows them to dissolve in water?
High volatility
Polar nature of water
High hardness
Low density
What happens to the lattice energy of an ionic compound as the charge of the ions increases?
It decreases
It increases
It remains the same
It fluctuates
Before you leave, take our quick quiz to enhance your learning!

