In AP Biology, understanding the structure and chemical properties of water is essential as it underpins many biological processes. Water‘s polarity and hydrogen bonding give it unique chemical properties, such as high specific heat, cohesion, and solvent abilities. These properties are crucial for maintaining life, influencing everything from cell function to climate regulation. Additionally, the phase changes between water and water vapor play a significant role in the Earth’s water cycle and energy balance.
Learning Objectives
By studying the structure and chemical properties of water in AP Biology, students will learn about water’s molecular structure, polarity, and hydrogen bonding. They will understand its unique chemical properties, such as high specific heat, cohesion, adhesion, and solvent capabilities. Students will explore how these properties support life processes, influence cellular functions, and regulate climate. Additionally, they will learn about the significance of water’s phase changes, including the transformation between water and water vapor.
Structure of Water
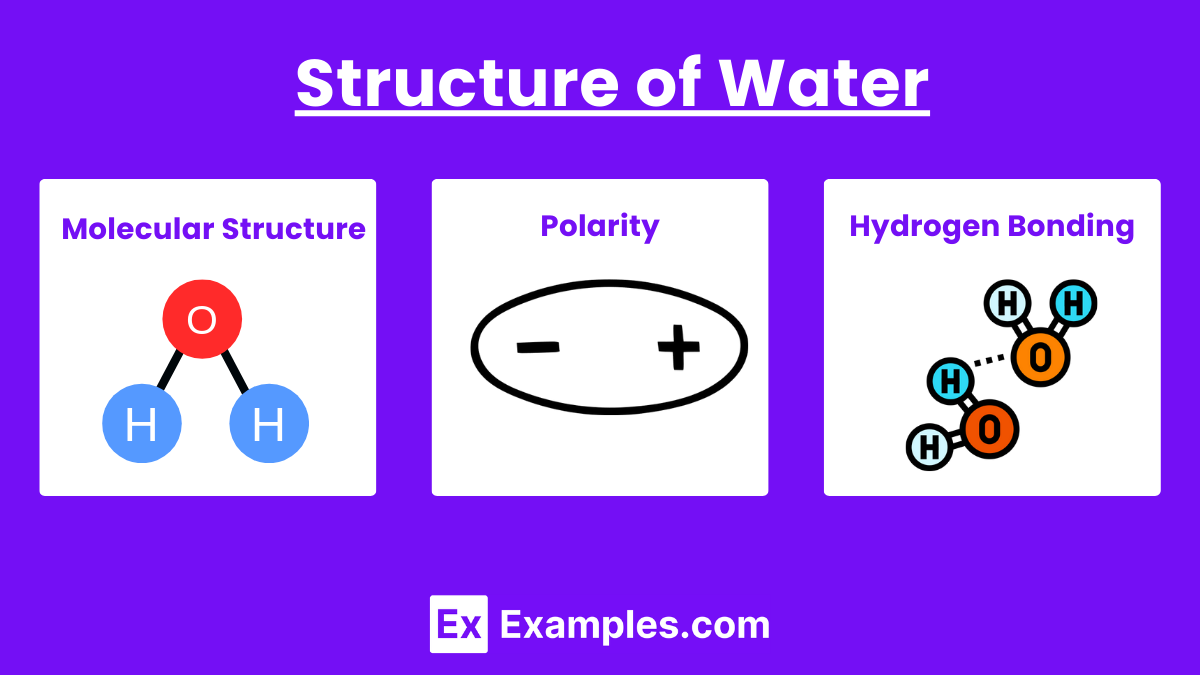
Molecular Structure
- Chemical Formula: H₂O
- Composition: Two hydrogen atoms covalently bonded to one oxygen atom.
- Molecular Shape: Bent or V-shaped due to the arrangement of electron pairs around the oxygen atom.
- Bond Angle: Approximately 104.5 degrees between the hydrogen-oxygen-hydrogen atoms.
Polarity
- Electronegativity: Oxygen is more electronegative than hydrogen, causing an unequal sharing of electrons.
- Partial Charges: Oxygen atom has a partial negative charge (δ-), while hydrogen atoms have partial positive charges (δ+).
- Dipole Moment: The separation of charges creates a dipole moment, making water a polar molecule.
Hydrogen Bonding
- Definition: Weak interactions between the hydrogen atom of one water molecule and the oxygen atom of another.
- Importance: Hydrogen bonds are responsible for many of water’s unique properties, such as high surface tension, specific heat capacity, and solvent abilities.
Chemical Properties of Water
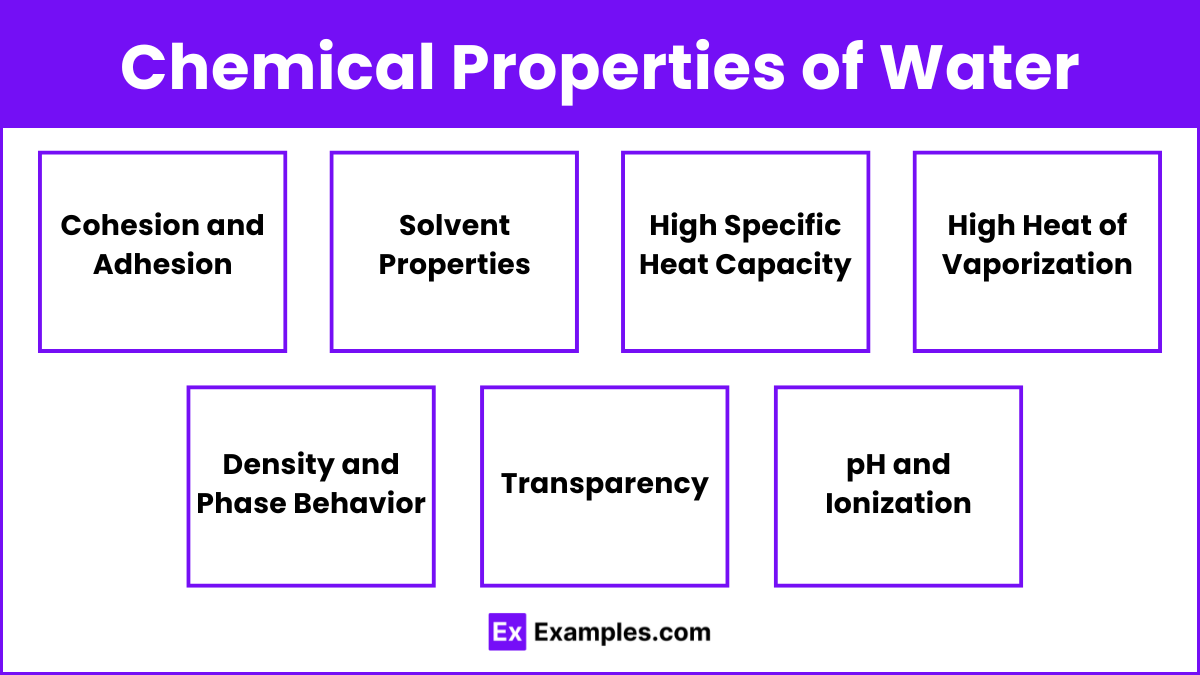
Cohesion and Adhesion
- Cohesion: Attraction between water molecules due to hydrogen bonding. Results in high surface tension, allowing insects to walk on water and droplets to form.
- Adhesion: Attraction between water molecules and other substances. Enables capillary action, which helps water move through plant vessels and soil pores.
Solvent Properties
- Universal Solvent: Water’s polarity allows it to dissolve many substances, making it a vital medium for chemical reactions in biological and environmental processes.
- Hydration Shells: Water molecules surround and interact with solute ions and molecules, stabilizing them in solution.
High Specific Heat Capacity
- Definition: The amount of heat required to raise the temperature of 1 gram of water by 1 degree Celsius.
- Significance: Water’s high specific heat capacity helps regulate temperature in organisms and environments, moderating climate and weather patterns.
High Heat of Vaporization
- Definition: The amount of energy needed to convert 1 gram of liquid water to gas at its boiling point.
- Significance: Evaporation of water requires significant energy, providing a cooling effect for organisms and influencing weather and climate.
Density and Phase Behavior
- Liquid State: Water is denser as a liquid than as a solid due to the arrangement of hydrogen bonds.
- Solid State: Ice forms a crystalline structure, making it less dense than liquid water, allowing it to float.
- Density Maximum: Water reaches its maximum density at 4 degrees Celsius, which plays a crucial role in aquatic ecosystems by affecting water stratification and circulation.
Transparency
- Definition: Water’s ability to transmit light, allowing sunlight to penetrate and support photosynthesis in aquatic ecosystems.
- Significance: Transparency is essential for the survival of aquatic plants and phytoplankton, which form the base of the food web.
pH and Ionization
- Autoionization: Water can self-ionize to form hydronium (H₃O⁺) and hydroxide (OH⁻) ions.
- pH Scale: A measure of the concentration of hydrogen ions (H⁺) in a solution. Pure water has a neutral pH of 7.
- Buffering Capacity: Water’s ability to resist changes in pH is vital for maintaining stable conditions in biological and environmental systems.
Biological Significance of Water Properties
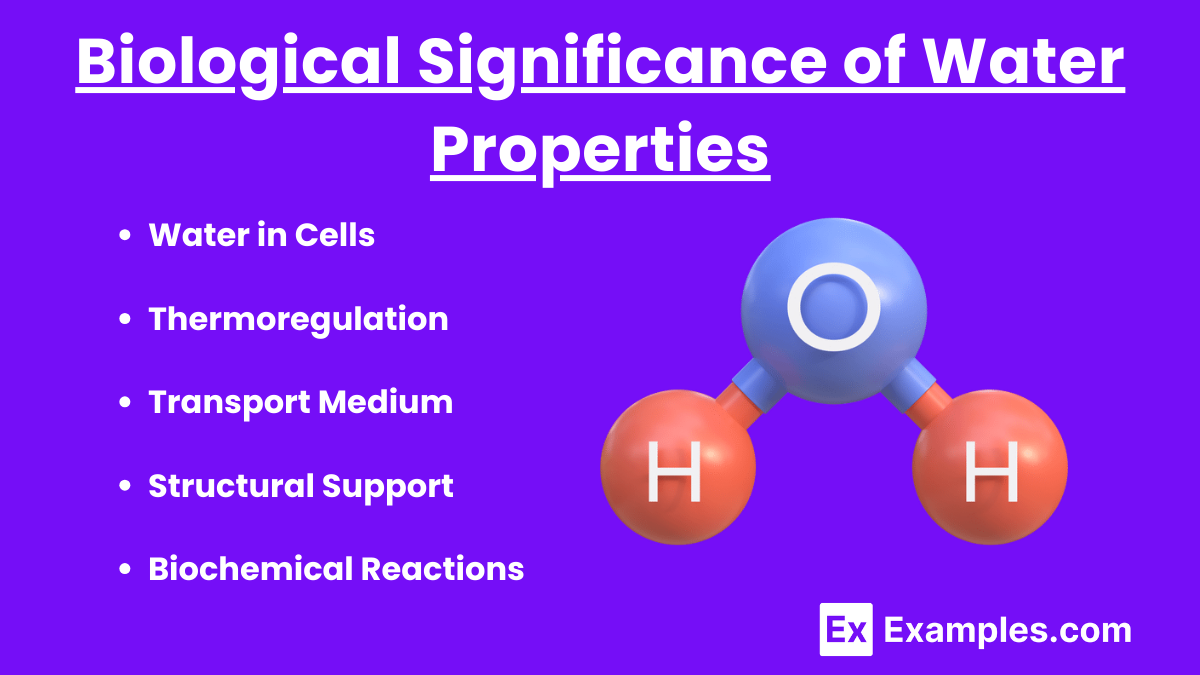
Water in Cells
- Cytoplasm: Water is the primary component of the cytoplasm, facilitating chemical reactions and nutrient transport.
- Solvent Abilities: Water dissolves ions and organic molecules, aiding in cellular processes and biochemical reactions.
Thermoregulation
- Homeostasis: Water’s high specific heat helps organisms maintain stable internal temperatures despite external fluctuations.
- Evaporative Cooling: Sweating and transpiration allow organisms to release excess heat through water evaporation.
Transport Medium
- Circulatory System: Water-based fluids like blood and lymph transport nutrients, gases, and waste products throughout the body.
- Plant Transport: Water moves through xylem and phloem, carrying nutrients and sugars in plants.
Structural Support
- Turgor Pressure: Water within plant cells creates turgor pressure, maintaining cell rigidity and structural integrity.
- Hydration Shells: Water molecules stabilize proteins and nucleic acids by forming hydration shells around them.
Biochemical Reactions
- Hydrolysis: Water participates in hydrolysis reactions, breaking down complex molecules into simpler ones.
- Condensation Reactions: Water is a product of condensation reactions, forming covalent bonds between molecules.
Environmental Significance of Water Properties
Water Cycle
- Processes: Evaporation, condensation, precipitation, infiltration, and runoff.
- Significance: Water’s unique properties drive the hydrological cycle, influencing climate, weather, and the distribution of water resources.
Aquatic Ecosystems
- Thermal Regulation: High specific heat capacity and heat of vaporization help stabilize temperatures in aquatic environments, supporting diverse life forms.
- Dissolved Oxygen: Water’s solvent properties enable the dissolution of oxygen, which is critical for the respiration of aquatic organisms.
- Nutrient Transport: Water’s polarity and solvent abilities facilitate the movement of nutrients and minerals in aquatic systems.
Soil and Plant Interactions
- Capillary Action: Adhesion and cohesion enable water to move through soil and plant tissues, supporting nutrient uptake and transpiration.
- Soil Moisture Retention: Water’s properties influence soil structure and its ability to retain moisture, affecting plant growth and agricultural productivity.
Climate Regulation
- Heat Distribution: Ocean currents and the heat capacity of water help distribute thermal energy around the planet, moderating global climate patterns.
- Evapotranspiration: The combined process of evaporation and plant transpiration affects humidity, precipitation, and weather systems.