Hypotonic vs. Hypertonic: What’s the Difference? With Examples
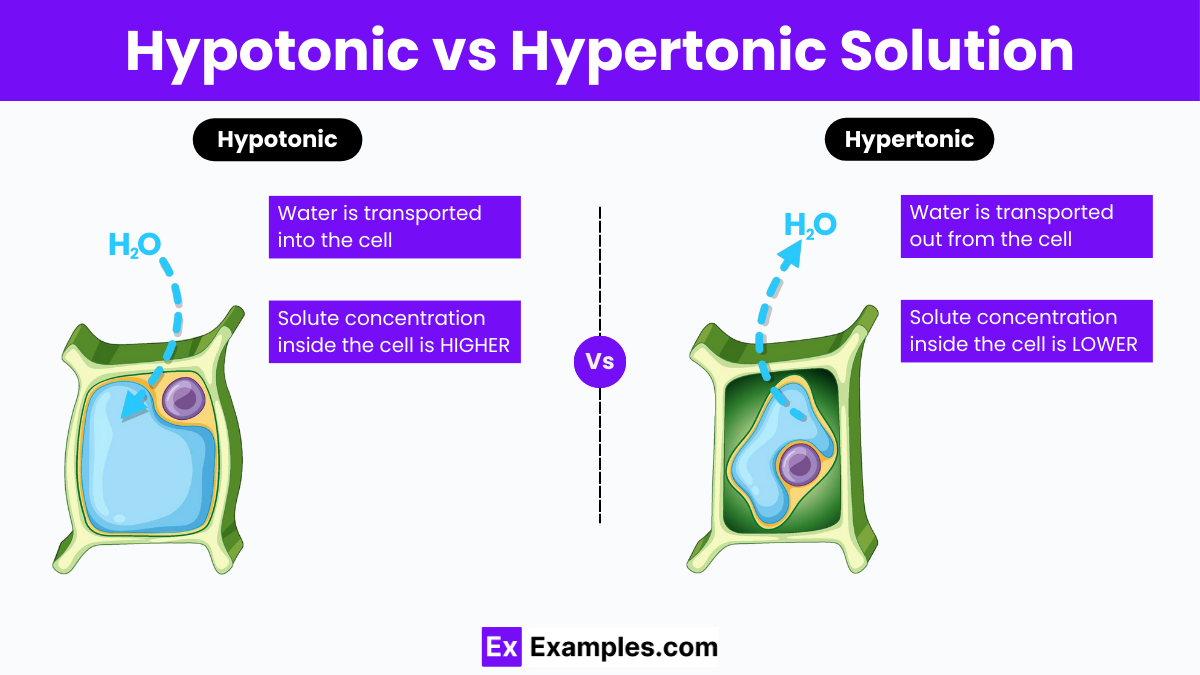
Hypotonic and hypertonic solutions are critical in determining how cells manage water intake and release. A hypotonic solution has a lower concentration of solutes compared to the cell’s interior, leading to water moving into the cell and causing it to swell. In contrast, a hypertonic solution contains a higher concentration of solutes than inside the cell, prompting water to flow out and the cell to shrink. This introduction highlights the fundamental differences between these solutions and their effects on cellular health.
What is Hypotonic?
A hypotonic solution refers to a solution that has a lower concentration of solutes (such as salts and sugars) compared to another solution, typically the fluid within living cells. In biological contexts, when cells are exposed to a hypotonic environment, the surrounding solution has less solute and more water compared to the inside of the cell. This difference creates an osmotic pressure gradient that causes water to move into the cell. As water enters, the cell swells and can potentially burst if the pressure becomes too great. This process is critical in maintaining cellular functions and is a key concept in understanding fluid balance in living organisms.
Examples of Hypotonic Solutions
- Tap Water: When you place a cell in tap water, the water is typically hypotonic compared to the fluid inside the cell. This causes water to flow into the cell, leading to swelling or even bursting if the cell is unable to expel the excess water quickly enough.
- Freshwater: Freshwater environments act as hypotonic solutions for aquatic organisms that live in saltwater. When these organisms are exposed to freshwater, their cells absorb water rapidly, which can disrupt their internal balance.
- Distilled Water: Often used in laboratory settings, distilled water provides an example of an extremely hypotonic solution because it contains no solutes. Cells placed in distilled water will swell as water rushes in to equalize the solute concentration inside and outside the cell.
What is Hypertonic?
A hypertonic solution is one that has a higher concentration of solutes compared to the fluid inside a cell. In this scenario, the external solution draws water out of the cell due to the osmotic pressure gradient, leading to cell shrinkage or crenation. Hypertonic solutions are commonly used in medical treatments to manage swelling and in food preservation, as their high solute concentration helps inhibit the growth of bacteria and other microorganisms by dehydrating them. Understanding the effects of hypertonic solutions is essential for applications ranging from healthcare to food safety.
Examples of Hypertonic Solutions
- Ocean Water: The high salt content of ocean water makes it hypertonic relative to the bodily fluids of most organisms. Cells exposed to ocean water lose water, causing them to shrink, which can be detrimental to freshwater organisms if they are not adapted to such conditions.
- Salt Solutions Used in Medical Treatments: Medical professionals often use saline solutions that are hypertonic to reduce swelling by drawing fluids out of swollen tissues. This application is common in managing edemas and other similar conditions.
- Sugar Syrups in Food Preservation: In food preservation, sugar syrups can create a hypertonic environment around fruits, causing microbial cells to lose water and thereby inhibiting their growth and spoilage activity. This technique is widely used in making preserved fruits and jams.
Differences Between Hypotonic and Hypertonic Solutions
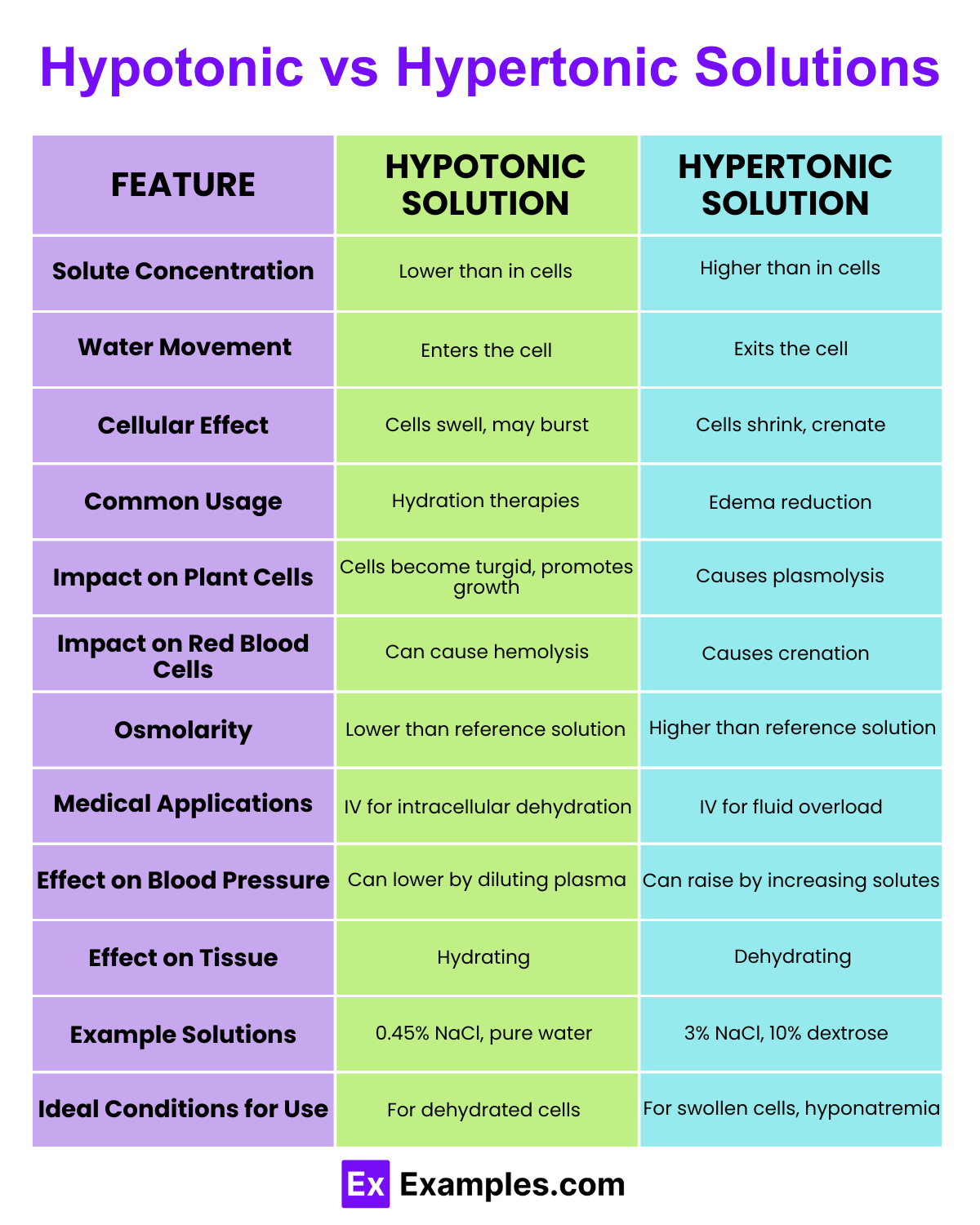
| Feature | Hypotonic Solution | Hypertonic Solution |
|---|---|---|
| Solute Concentration | Lower than that of the cell’s cytoplasm. | Higher than that of the cell’s cytoplasm. |
| Water Movement | Water moves into the cell. | Water moves out of the cell. |
| Cellular Effect | Causes cells to swell and can lead to bursting. | Causes cells to shrink and can lead to crenation. |
| Osmotic Pressure | Lower osmotic pressure outside the cell. | Higher osmotic pressure outside the cell. |
| Common Usage | Used to rehydrate cells and tissues. | Used to reduce edema and stabilize excess hydration. |
| Examples | Freshwater environments for aquatic organisms. | Saltwater environments for marine organisms. |
| Impact on Plant Cells | Cells become turgid (stiff and firm). | Cells become plasmolyzed (membrane pulls away from wall). |
| Impact on Animal Cells | Cells may lyse (burst) due to excessive swelling. | Cells undergo plasmolysis, shrinking from the membrane. |
| Medical Relevance | Administering IV solutions that are less concentrated than blood plasma. | Administering IV solutions that are more concentrated than blood plasma. |
| Physiological Response | Can lead to cytolysis in extreme cases. | Often results in dehydration of cells. |
| Tonicity | The relative concentration of solutions that determine the direction and extent of diffusion. | The relative concentration of solutions that affect cellular dehydration or crenation. |
| Effect on Blood Cells | Red blood cells swell and may burst (hemolysis). | Red blood cells shrink (crenate). |
| Clinical Examples | Hydration therapy for patients with high electrolyte levels. | Hypertonic saline in treating severe hyponatremia. |
| Environmental Impact | Vital for supporting life in hypotonic freshwater habitats. | Necessary for organisms adapted to hypertonic marine environments. |
| Impact on Microorganisms | Promotes rapid growth in hypotonic conditions. | Can inhibit bacterial growth due to osmotic pressure. |
| Biological Significance | Crucial for the absorption of nutrients and minerals in plants. | Important for the conservation of water in organisms living in saline environments. |
Key Similarities Between Hypotonic and Hypertonic Solutions
When discussing the behavior of cells in different solutions, hypotonic and hypertonic solutions are two crucial concepts in the study of osmosis and cellular behavior. Despite their differences in how they affect cells, these two types of solutions share several key similarities:
1. Involvement in Osmosis
Both hypotonic and hypertonic solutions are directly involved in the process of osmosis. Osmosis is the movement of water across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. In both cases, the direction and rate of water movement are governed by the gradient in solute concentration across the cell membrane.
2. Impact on Cellular Water Movement
In both hypotonic and hypertonic solutions, the primary effect is on the movement of water into or out of the cell. This movement is crucial for maintaining cell structure and function. In a hypotonic solution, water moves into the cell, potentially causing it to swell and burst. In a hypertonic solution, water moves out of the cell, which can lead to cell shrinkage or crenation.
3. Dependence on Solute Concentrations
The effect of both hypotonic and hypertonic solutions on cells depends on the concentration of solutes outside the cell compared to the inside. The difference in solute concentration determines the direction of water movement, influencing cell survival and behavior.
4. Relevance to Biological Functions
Both types of solutions are relevant in various biological functions and medical applications. For example, understanding these solutions helps in managing dehydration, administering intravenous fluids, and designing therapies for maintaining cellular health in different environmental conditions.
5. Use in Experimental and Clinical Settings
Researchers and clinicians use both hypotonic and hypertonic solutions to study and manipulate cell behavior and physiology. These solutions are tools for experiments in laboratories to understand osmoregulation, cell volume regulation, and the effects of solute concentration on cellular processes.
FAQs
What is the Difference Between Hypotonic and Hypertonic Solution?
A hypotonic solution has fewer solutes than a cell, causing water to enter the cell, while a hypertonic solution has more, drawing water out.
Does Hypertonic Shrink or Swell?
A hypertonic solution causes cells to shrink as water moves out of the cell to balance solute concentrations.
What is the Difference Between Hypertonic and Hypotonic Hydration?
Hypotonic hydration swells cells by promoting water intake, whereas hypertonic hydration shrinks them by drawing water out.
How Do You Remember Hypertonic and Hypotonic?
Remember: “Hyper” means over (more solutes outside, cells shrink); “Hypo” means under (fewer solutes outside, cells swell).
Is 10% NaCl Hypertonic or Hypotonic?
A 10% NaCl solution is hypertonic, containing a higher solute concentration compared to most living cells, causing them to shrink.