Which of the following units is NOT a unit of surface tension?
N/m
dyne/cm
J/m²
kg/m²


Unit of surface tension is a crucial concept in physics, defining the force per unit length required to overcome the cohesive forces between molecules at the surface of a liquid. It’s measured in various units depending on the system of measurement, such as Newtons per meter (N/m) in the International System of Units (SI) or dynes per centimeter (dyn/cm) in the CGS (Centimeter-Gram-Second) system. Understanding surface tension is fundamental in explaining phenomena like capillary action, droplet formation, and the behavior of liquids in various environments, making it essential in physics and numerous practical applications.
Where:
Surface tension is a measure of the force per unit length acting along the surface of a liquid, caused by the cohesive forces between its molecules. In the International System of Units (SI), surface tension is quantified in Newtons per meter (N/m). This unit represents the amount of force required to stretch or deform the surface of a liquid by one meter. It’s a crucial concept in physics and chemistry, influencing phenomena such as droplet formation, capillary action, and the behavior of fluids in various applications.
In the CGS system, surface tension is measured in dynes per centimeter (dyn/cm). This unit represents the force exerted perpendicular to a line of one centimeter at the surface of a liquid. Dynes are the unit of force, and centimeters are the unit of length in the CGS system. Surface tension quantifies the cohesive forces between molecules at the surface of a liquid, influencing various phenomena such as droplet formation, wetting behavior, and capillary rise.
| Aspect | Unit of Surface Tension | Unit of Surface Energy |
|---|---|---|
| Definition | Force per unit length | Energy per unit area |
| Symbol | N/m (SI), dyn/cm (CGS) | J/m² (SI), erg/cm² (CGS) |
| Physical Interpretation | Tendency to minimize surface area | Ability to do work at the surface |
| Measurement | Measures forces at the surface | Measures energy state of the surface |
| Applications | Capillary action, droplet formation, wetting behavior | Material strength, adhesion, surface reactions |
| Unit | Abbreviation | System | Approximate Value (for water at 20°C) |
|---|---|---|---|
| Newtons per meter | N/m | International System of Units (SI) | 0.0728 N/m |
| Dynes per centimeter | dyn/cm | Centimeter-Gram-Second (CGS) system | 72.8 dyn/cm |
| Pounds per inch | lb/in | Imperial system | 0.413 lb/in |
| Millinewtons per meter | mN/m | SI-derived unit | 72.8 mN/m |
| Kilograms per second squared | kg/s² | Alternative SI-derived unit | 72.8 N/m |
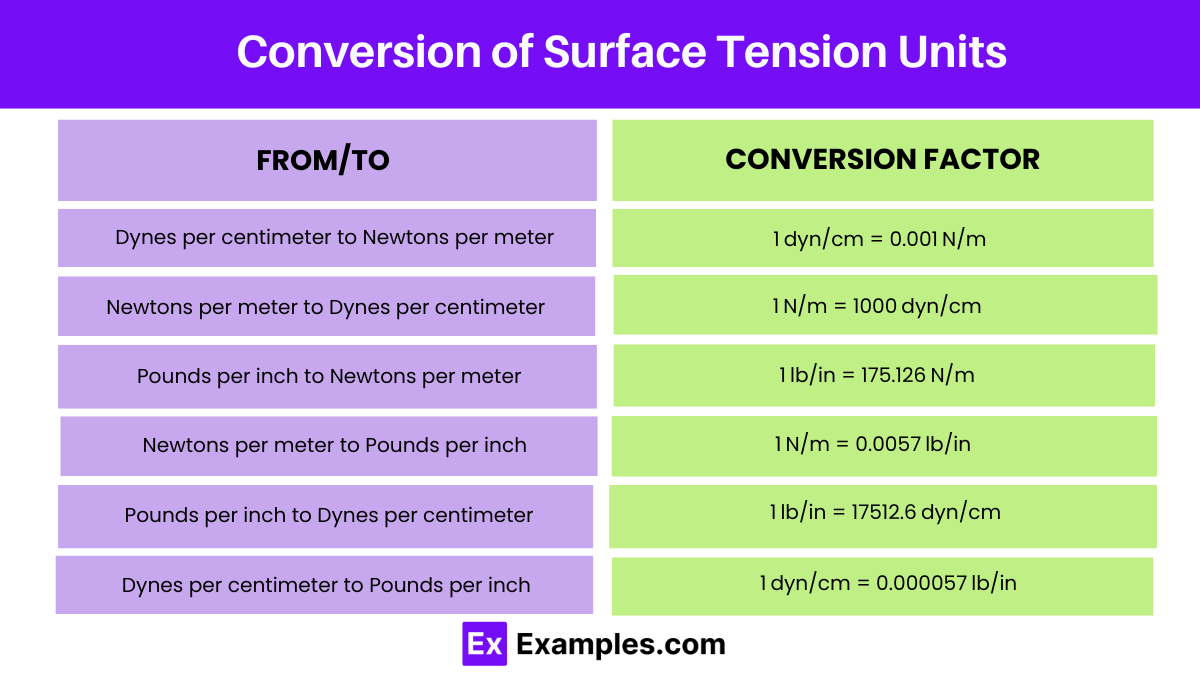
| From (Unit) / To (Unit) | Conversion Factor | Example |
|---|---|---|
| Dynes per centimeter to Newtons per meter | 1 dyn/cm = 0.001 N/m | 10 dyn/cm = 0.01 N/m |
| Newtons per meter to Dynes per centimeter | 1 N/m = 1000 dyn/cm | 10 N/m = 10,000 dyn/cm |
| Pounds per inch to Newtons per meter | 1 lb/in = 175.126 N/m | 10 lb/in = 1751.26 N/m |
| Newtons per meter to Pounds per inch | 1 N/m = 0.0057 lb/in | 10 N/m = 0.057 lb/in |
| Pounds per inch to Dynes per centimeter | 1 lb/in = 17512.6 dyn/cm | 10 lb/in = 175126 dyn/cm |
| Dynes per centimeter to Pounds per inch | 1 dyn/cm = 0.000057 lb/in | 10 dyn/cm = 0.00057 lb/in |
Surface tension is responsible for the ability of small insects to walk on water, the formation of raindrops, the shape of liquid droplets, and the functioning of soap bubbles.
Water, mercury, and liquid metals typically exhibit high surface tension due to the strong cohesive forces between their molecules.
Understanding surface tension is crucial in various scientific and engineering fields, including fluid mechanics, materials science, biology, and chemistry, where it influences processes such as adhesion, coating, and fluid behavior.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which of the following units is NOT a unit of surface tension?
N/m
dyne/cm
J/m²
kg/m²
Which of the following is a correct unit of surface tension?
Pascal
Newton
Joule per meter squared
Watt
The surface tension of water is commonly measured in:
N/s
N/m
N/kg
N/m²
Which unit can be used interchangeably with N/m for measuring surface tension?
J/m
J/m²
J/m³
J/s
If the surface tension of a liquid is 72.8 mN/m, what is it in dyne/cm?
7.28 dyne/cm
72.8 dyne/cm
728 dyne/cm
0.728 dyne/cm
What is the SI unit of surface tension?
Pascal
Newton per meter
Dyne per centimeter
Joule per second
Surface tension can also be represented in terms of energy as:
Joule per cubic meter
Joule per square meter
Newton per second
Newton per square meter
A liquid has a surface tension of 0.072 J/m². What is this value in N/m?
0.072 N/m
0.72 N/m
7.2 N/m
72 N/m
Identify the incorrect unit of surface tension from the following:
N/m
dyne/cm
J/m²
N/m²
What does a surface tension value of 73 dyne/cm convert to in N/m?
0.073 N/m
7.3 N/m
0.73 N/m
73 N/m
Before you leave, take our quick quiz to enhance your learning!

