What is the SI unit of energy?
Calorie
Watt
Joule
Electronvolt
In physics, we often discuss different quantities like energy, time, and work. Each of these quantities needs a standard unit of measurement to help us describe and understand them properly. For example, when we say someone weighs 36 kilograms (kg) and lives 1200 kilometers (km) away, “kg” and “km” are the units used to describe weight and distance, respectively. Similarly, we use Kelvin to measure temperature.
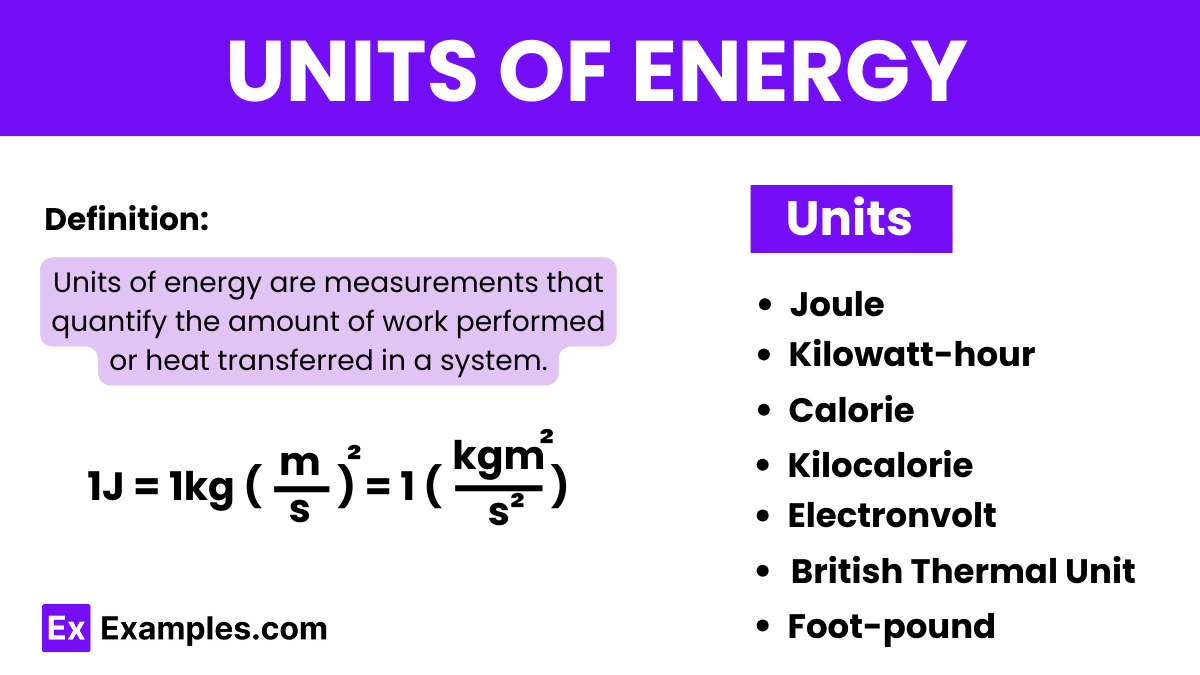
The Joule (symbol: J) is the official unit of energy in the International System of Units (SI). It is named after the English physicist James Prescott Joule. One joule represents the amount of energy transferred when applying a force of one newton over a distance of one meter.
Common Use: Joules are used universally in all fields of science and engineering to measure energy, work, or heat. For example, in electrical terms, one joule is the energy expended by passing an electric current of one ampere through a resistance of one ohm for one second.
The erg is the unit of energy in the centimeter-gram-second (CGS) system of units. It is a smaller unit compared to the joule. One erg is defined as the amount of energy done by a force of one dyne exerted for a distance of one centimeter.
Common Use: Though largely replaced by the joule in many fields due to the international adoption of the SI system, the erg is still used in some contexts within physics, especially in electromagnetism and astronomy.
In the MKS (meter-kilogram-second) system, which is a precursor to the modern SI system, the unit of energy is also the joule. The MKS system uses meters, kilograms, and seconds as its basic units of length, mass, and time, respectively. This makes the joule’s definition in MKS the same as in SI.
Common Use: The MKS system was widely used before the official adoption of the SI system and its use of the joule paved the way for its inclusion in the SI system. Today, joules are universally recognized and used across scientific and engineering disciplines.
| Energy Unit | Symbol |
|---|---|
| Joule | J |
| Kilowatt-hour | kWh |
| Calorie | cal |
| Kilocalorie | kcal |
| Electronvolt | eV |
| British Thermal Unit | BTU |
| Foot-pound | ft-lb |
The Joule is the SI unit of energy named after James Prescott Joule. It measures the amount of energy expended when a force of one newton is applied over a distance of one meter. It’s used universally in science and engineering to quantify energy, work, or heat.
This unit is widely used in electrical applications, particularly for measuring electrical energy usage in homes and businesses. It represents the energy produced or consumed by a power of one kilowatt over a period of one hour.
A calorie is a unit of energy that originally was defined as the amount of heat needed to raise the temperature of one gram of water by one degree Celsius at atmospheric pressure. It’s commonly used in chemistry and the food and beverage industry to specify the energy content.
Also known as a food calorie, kilocalories are used to express the energy content in foods and are equivalent to 1000 small calories. In dietary contexts, these are the “calories” that are counted to determine energy intake.
An electronvolt is a unit of energy equal to approximately 1.602×10−191.602×10−19 joules. It is commonly used in atomic, nuclear, and particle physics to describe the energy levels of electrons and other subatomic particles.
The BTU is used primarily in the United States for heating and cooling systems and defines the amount of energy needed to raise the temperature of one pound of water by one degree Fahrenheit. It is still common in industrial and commercial heating and cooling.
This unit measures energy in terms of mechanical work done when a force of one pound-force is exerted along one foot of displacement. It is mainly used in the United States and remains popular in mechanical engineering and physics for measuring rotational forces.
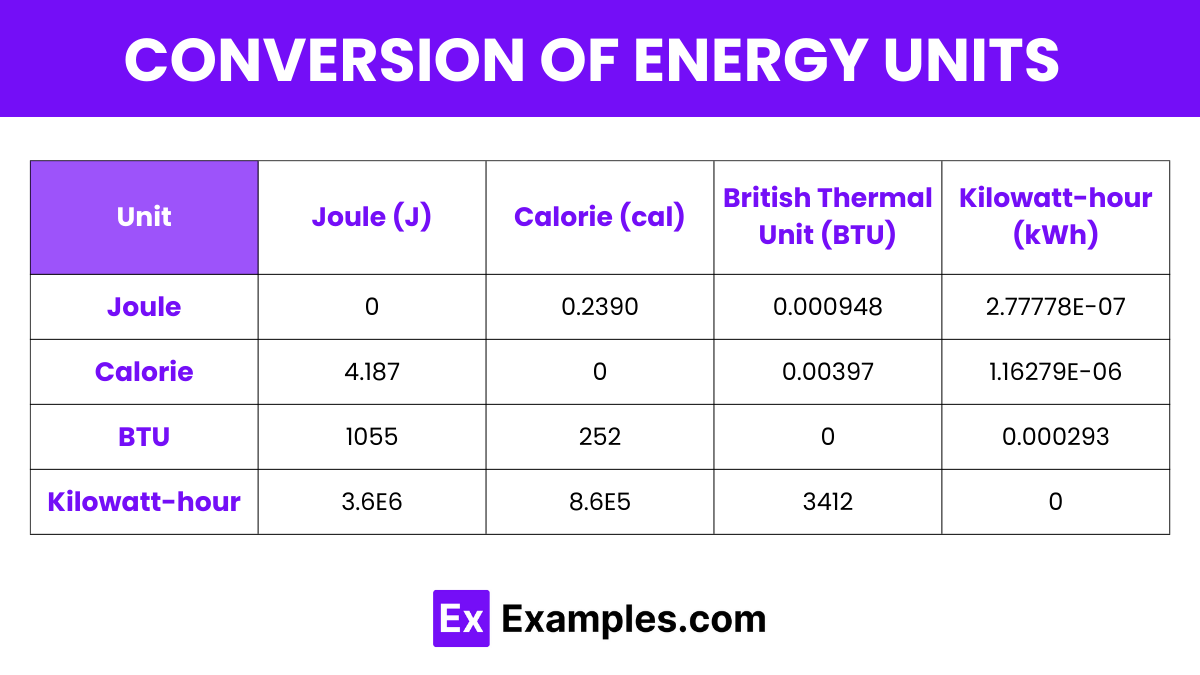
| Unit | Joule (J) | Calorie (cal) | British Thermal Unit (BTU) | Kilowatt-hour (kWh) |
|---|---|---|---|---|
| Joule | 0 | 0.2390 | 0.000948 | 2.77778E-07 |
| Calorie | 4.187 | 0 | 0.00397 | 1.16279E-06 |
| BTU | 1055 | 252 | 0 | 0.000293 |
| Kilowatt-hour | 3.6E6 | 8.6E5 | 3412 | 0 |
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the SI unit of energy?
Calorie
Watt
Joule
Electronvolt
Which unit of energy is commonly used in the food industry?
Joule
Calorie
Kilowatt-hour
Electronvolt
1 kilowatt-hour (kWh) is equivalent to how many joules?
1,000 J
3,600 J
36,000 J
3,600,000 J
What unit of energy is used in nuclear physics to measure small energy levels?
Joule
Calorie
Electronvolt
Erg
Which of the following is NOT a unit of energy?
Joule
Newton
Calorie
Kilowatt-hour
How many joules are in a calorie?
4.184 J
3.6 J
1.0 J
0.239 J
Which unit is larger, a megajoule (MJ) or a gigajoule (GJ)?
Megajoule
Gigajoule
They are equal
Depends on the context
Which unit is used to measure the energy produced by burning fuels?
Joule
Calorie
British Thermal Unit (BTU)
Electronvolt
How many kilojoules (kJ) are in a megajoule (MJ)?
10
100
1,000
1,0000
Which unit of energy is equivalent to 1 Newton-meter?
Joule
Calorie
Watt
Electronvolt
Before you leave, take our quick quiz to enhance your learning!

