What is the primary driving force behind diffusion?
Electrical gradients
Pressure differences
Concentration gradients
Temperature gradients
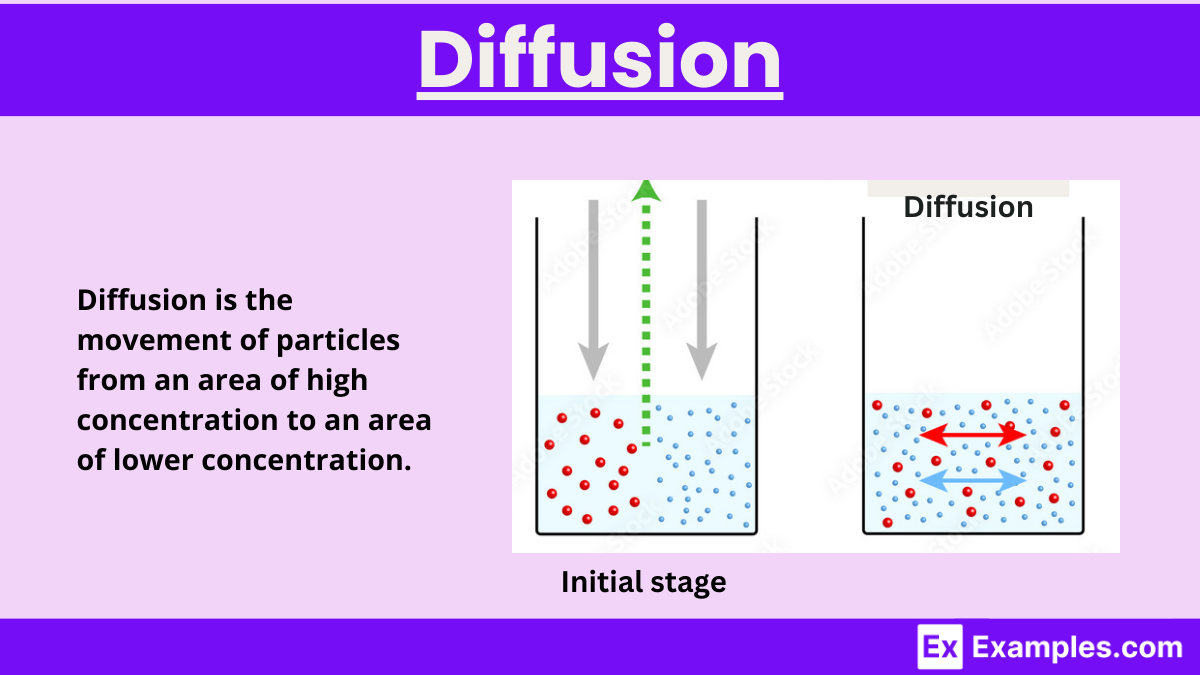
Diffusion is the natural movement of particles from an area of high concentration to one of low concentration, driven by differences in concentration or energy levels. This principle is universal, applying not only to physical and chemical processes but also to the spread of ideas, data, and financial trends. Remarkably, diffusion can also occur “uphill,” defying the usual flow, in specific scenarios. Its inherent randomness makes it a key concept in fields as varied as sociology, economics, and data science, illustrating the broad impact of this fundamental process.
Diffusion is a fundamental process through which particles move from an area of higher concentration to an area of lower concentration. This movement is driven by the natural desire of particles to occupy space more uniformly and achieve equilibrium. The concept of diffusion is widely applicable across various fields, including physics, chemistry, biology, and even social sciences.
Diffusion stands out as a core principle in biology, crucial for grasping how molecules move within and between cells. This process naturally enables particles to migrate from areas of high concentration to those of low concentration, leading to an even distribution. The kinetic energy of the particles drives this movement until they achieve equilibrium, meaning the particle concentration becomes uniform throughout the space.
Fill a clear glass with water and carefully add a single drop of ink to the center without stirring. Watch as the ink slowly spreads throughout the water, creating a swirl of color. This process demonstrates diffusion in action, as the ink molecules move from an area of high concentration (the drop) to lower concentration areas (the surrounding water) until they are evenly dispersed. This experiment visually showcases how diffusion works, making it easier to understand the concept.
Spraying perfume in a room disperses its scent everywhere, demonstrating how perfume molecules move from a concentrated area to fill the entire space.
A tea bag in hot water shows diffusion as the tea’s flavors spread evenly throughout the water.
Dissolving sugar in water is an example of diffusion, where sugar molecules spread uniformly in the water.
Oxygen moving from the lungs into the blood and carbon dioxide moving out showcases diffusion in the human body.
A hot drink cools down as its heat diffuses into the cooler surrounding air.
Simple diffusion involves the direct movement of molecules across a permeable membrane without the need for energy. Molecules move from an area of high concentration to an area of low concentration until they reach equilibrium. An everyday example of simple diffusion is the exchange of oxygen and carbon dioxide in our lungs.
Facilitated diffusion is similar to simple diffusion but requires the assistance of carrier proteins. These proteins help specific molecules, which cannot directly pass through the cell membrane, to move across. Glucose entering a cell with the help of insulin is a classic example of facilitated diffusion.
Osmosis is a specialized type of diffusion that involves the movement of water molecules. In osmosis, water moves across a semipermeable membrane from a less concentrated solution to a more concentrated one. A practical illustration of osmosis is the absorption of water by plant roots from the soil.
Channel diffusion refers to the movement of ions and small molecules through water-filled protein channels in the cell membrane. These channels are specific to certain substances and allow them to pass through more easily. For instance, potassium ions move through potassium channels in nerve cells to transmit signals.
Diffusion, the process by which particles move from an area of high concentration to an area of low concentration, is influenced by several factors. Understanding these factors helps in comprehending how diffusion occurs in different environments and systems.
The concentration gradient is the difference in concentration between two areas. A higher gradient results in a faster rate of diffusion as particles move more rapidly to achieve equilibrium.
Temperature plays a crucial role in diffusion. Higher temperatures increase the kinetic energy of particles, making them move faster. This acceleration enhances the rate of diffusion.
The size of the particles involved in diffusion affects the rate at which they move. Smaller particles, having less mass, diffuse faster than larger ones.
The medium through which diffusion occurs significantly impacts the rate of diffusion. For instance, gases diffuse faster than liquids because particles in a gas are farther apart, allowing easier movement.
In biological systems, the thickness of the cell membrane through which diffusion occurs can affect the rate. Thinner membranes allow for quicker diffusion.
A larger surface area facilitates a higher rate of diffusion. This is particularly noticeable in biological systems where structures like the alveoli in the lungs have a large surface area to maximize the diffusion of oxygen and carbon dioxide.
Concentration Gradient: Diffusion occurs due to differences in concentration. Substances move from areas of high concentration to areas of lower concentration until equilibrium is reached, driven by the gradient.
Temperature: Higher temperatures increase the kinetic energy of particles, accelerating diffusion. This is because particles move more rapidly, spreading out more quickly across a space.
Particle Size: Smaller particles diffuse faster than larger ones. This is due to less resistance in their movement through the medium, allowing quicker distribution.
Medium Density: Diffusion is slower in denser mediums as particles encounter more resistance. In less dense mediums, particles move more freely, facilitating faster diffusion.
Distance: The greater the distance, the slower the diffusion. This is because particles have to travel a longer path to reach equilibrium, which takes more time.
Diffusion is the movement of particles from an area of high concentration to low concentration, resulting in an even distribution.
In biology, diffusion is essential for transporting nutrients and waste products in and out of cells.
Diffusion involves solutes moving through a solution, while osmosis is the diffusion of water across a semipermeable membrane.
In anatomy, diffusion refers to the passive transport of molecules like oxygen and carbon dioxide across cell membranes.
During respiration, diffusion enables oxygen to move from the lungs into blood and carbon dioxide out.
An example of diffusion in biology is the exchange of oxygen and carbon dioxide gases in the lungs.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary driving force behind diffusion?
Electrical gradients
Pressure differences
Concentration gradients
Temperature gradients
Which of the following is an example of diffusion in the human body?
Blood flow in veins
Oxygen moving from alveoli to blood
Muscle contraction
Heartbeat
Which factor does NOT affect the rate of diffusion?
Temperature
Size of molecules
Concentration gradient
Color of molecules
Diffusion can occur in which of the following states of matter?
Solids only
Liquids only
Gases only
Solids, liquids, and gases
In diffusion, particles move:
From low concentration to high concentration
From high concentration to low concentration
Randomly with no net movement
In fixed pathways
Which of the following best describes simple diffusion?
Movement of water through a semipermeable membrane
Movement of molecules through protein channels
Movement of molecules without the assistance of membrane proteins
Movement of ions against their concentration gradient
How does temperature affect the rate of diffusion?
Higher temperatures decrease the rate of diffusion
Higher temperatures increase the rate of diffusion
Temperature has no effect on diffusion
Lower temperatures increase the rate of diffusion
What is facilitated diffusion?
Diffusion of water through a membrane
Diffusion of molecules with the help of transport proteins
Active transport of ions
Diffusion that requires energy input
Which statement about diffusion is true?
It requires energy from ATP
It only occurs in living cells
It can move substances up their concentration gradient
It results in an even distribution of molecules
What happens to the rate of diffusion as the concentration gradient decreases?
The rate increases
The rate decreases
The rate remains constant
The rate becomes zero immediately
Before you leave, take our quick quiz to enhance your learning!

