What is the term for the smallest unit of matter that retains the chemical properties of an element?
Molecule
Atom
Ion
Compound

In Chemistry, everything around us is made up of tiny building blocks called particles. These particles can be atoms, molecules, or ions, and they are incredibly small, often invisible to the naked eye. Atoms are the basic units of matter and combine to form molecules, which make up different substances. Ions are charged particles that form when atoms gain or lose electrons. Understanding particles helps us grasp how substances interact, change, and form new materials.
To find the number of particles in a substance, we use the formula:
This formula tells us that if we know how many moles of a substance we have, we can multiply that number by Avogadro’s number (6.022 x 10²³) to get the total number of particles. Avogadro’s number is a constant that represents the number of atoms, molecules, or ions in one mole of a substance. This calculation is essential for understanding and measuring the amount of matter in chemistry.
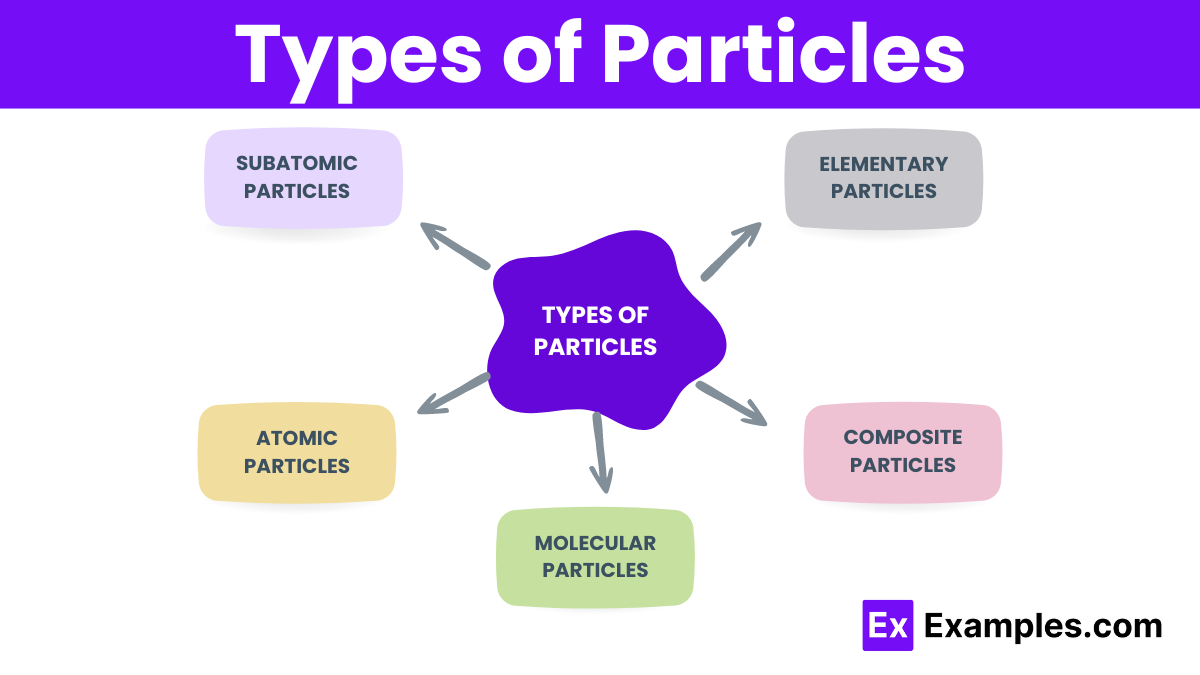
Subatomic particles are the fundamental constituents of atoms. They include:
Atomic particles refer to entire atoms and ions. They include:
Molecular particles are groups of atoms bonded together. They include:
Composite particles are particles made up of other particles. They include:
Elementary particles are the most basic building blocks of matter, not made of other particles. They include:
The size of a particle varies widely depending on the type of particle. Subatomic particles like protons, neutrons, and electrons are incredibly small, with sizes on the order of femtometers (1 femtometer = 10⁻¹⁵ meters). Atoms, which are made up of these subatomic particles, are larger, typically around 0.1 to 0.5 nanometers (1 nanometer = 10⁻⁹ meters) in diameter. Molecules, which consist of two or more atoms bonded together, can range from less than a nanometer to several nanometers in size.
In a liquid, the particles (atoms or molecules) are closely packed together but not as tightly as in a solid. They have more freedom to move around, which allows liquids to flow and take the shape of their container. The particles in a liquid are in constant, random motion and can slide past each other. This movement is due to the moderate kinetic energy that liquid particles possess. The intermolecular forces in a liquid are strong enough to keep the particles close but not so strong as to prevent movement. This balance gives liquids their characteristic properties, such as viscosity (resistance to flow) and surface tension.
Particles in a solid are tightly packed together in a fixed, orderly arrangement. They are held in place by strong intermolecular forces, which give solids a definite shape and volume. The particles in a solid vibrate but do not move from their fixed positions, which explains why solids are rigid and incompressible. The lack of movement among particles in a solid means they have low kinetic energy compared to liquids and gases. There are different types of solids, such as crystalline solids, where particles are arranged in a repeating pattern, and amorphous solids, where the arrangement is more random.
Particles in a gas move freely and rapidly in all directions. They have much more kinetic energy compared to particles in solids or liquids, which allows them to overcome intermolecular forces and spread out. This movement causes gas particles to fill any container they are in completely. The particles collide with each other and the walls of the container, creating pressure. Because of the large spaces between them, gas particles can be compressed easily, and they expand to fill any available space. Understanding the behavior of gas particles helps explain properties like pressure, volume, and temperature in gases.
An atom consists of a nucleus and electrons. The nucleus, at the center of the atom, contains protons and neutrons, which are tightly bound together by the strong nuclear force. Protons have a positive charge, and neutrons have no charge. Surrounding the nucleus are electrons, which are negatively charged and occupy regions of space called electron clouds or orbitals. Electrons are much smaller and lighter than protons and neutrons and are held in orbit around the nucleus by the electrostatic force of attraction between the positively charged nucleus and the negatively charged electrons. The arrangement of these particles determines the atom’s properties and its ability to form bonds with other atoms.
A particle is a small piece of matter that makes up everything in the universe. Particles can be atoms, molecules, or subatomic particles like protons, neutrons, and electrons.
Show diagrams and models of atoms, molecules, and ions.
Conduct simple experiments to illustrate particle behavior in solids, liquids, and gases.
Explain how particles are present in common objects and substances.
Use simulations and interactive tools to visualize particle movement.
Break down complex ideas into simple, easy-to-understand terms.
Foster a classroom environment where students feel comfortable asking questions.
Compare particle behavior to familiar situations, like crowded rooms for gas particles.
Provide opportunities for students to build their own models of particles.
Reinforce key concepts with frequent reviews and quizzes.
Show how particle behavior affects real-world phenomena, such as weather patterns and chemical reactions.
The graviton is a hypothetical particle believed to carry the force of gravity in quantum theories, though it has not been experimentally observed.
The three general types of particles are subatomic particles (protons, neutrons, electrons), atoms, and molecules.
Currently, there is no confirmed particle smaller than a quark. Quarks are considered fundamental particles in the Standard Model of particle physics.
Yes, humans are made of atoms, which contain subatomic particles like electrons and protons, all governed by quantum mechanics.
Quantum particles include electrons, protons, neutrons, photons, quarks, and gluons, all following the principles of quantum mechanics.
Light exhibits both wave-like and particle-like properties, a concept known as wave-particle duality, where photons represent its particle aspect.
Understanding particles is essential for studying and explaining the properties and behaviors of all matter in the universe.
Photons are particles of light that exhibit both wave-like and particle-like properties.
Particles interact through fundamental forces: gravitational, electromagnetic, strong nuclear, and weak nuclear forces.
Quarks are fundamental particles that combine to form protons and neutrons within an atomic nucleus.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the term for the smallest unit of matter that retains the chemical properties of an element?
Molecule
Atom
Ion
Compound
What particle in an atom has a positive charge?
Electron
Neutron
Proton
Photon
What is the charge of an electron?
Positive
Neutral
Negative
Undefined
Which particle is found in the nucleus of an atom and has no charge?
Proton
Neutron
Electron
Positron
What particle is responsible for the chemical bonds between atoms?
Neutron
Proton
Electron
Muon
Which particle has the smallest mass?
Proton
Neutron
Electron
Neutrino
What is the mass number of an atom?
Number of electrons
Number of neutrons
Sum of protons and neutrons
Sum of protons and electrons
What type of particle is a neutrino?
Charged
Massless
Neutral and nearly massless
Heavy
What is an isotope?
Atoms with different numbers of protons
Atoms with the same number of neutrons
Atoms with the same number of protons but different numbers of neutrons
Atoms with different numbers of electrons
What term describes particles that are constituents of atomic nuclei?
Leptons
Baryons
Mesons
Quarks
Before you leave, take our quick quiz to enhance your learning!

