Which of the following is a common use for sodium hydroxide (NaOH)?
Food preservative
Cleaning agent
Fertilizer
Glass production


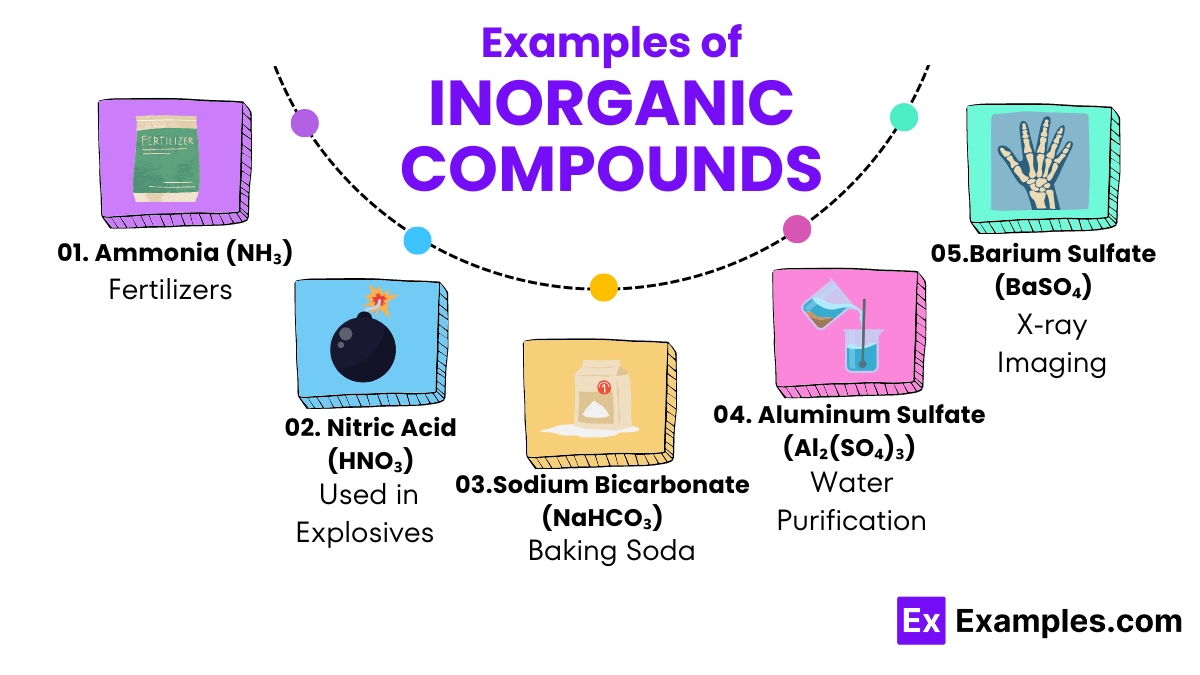
Inorganic compounds are substances that generally do not contain carbon-hydrogen bonds, which sets them apart from organic compounds in chemistry. Found abundantly in nature and commonly used in various industries, these compounds include minerals, salts, metals, and gases such as carbon dioxide and ammonia. Unlike organic compounds, which are often associated with living organisms, inorganic compounds are typically derived from non-living sources. Their wide range of applications, from building materials to essential nutrients, makes them crucial in both ecological systems and human industries.
The structure of inorganic compounds can vary widely, but they generally consist of a simple combination of atoms held together by ionic or covalent bonds. Ionic bonds occur between a metal and a non-metal, where electrons are transferred from one atom to another, creating charged ions that attract each other. For example, table salt (NaCl) is formed from sodium (Na) and chlorine (Cl) ions. Covalent bonds, on the other hand, involve the sharing of electrons between atoms, typically seen in compounds like water (H₂O), where oxygen shares electrons with hydrogen atoms.
| Property | Description |
|---|---|
| Melting Point | The temperature at which a solid becomes a liquid |
| Boiling Point | The temperature at which a liquid turns to vapor |
| Solubility | How well a substance dissolves in a solvent |
| Density | The mass per unit volume of a substance |
| Color | The color of the compound in solid or solution form |
| Odor | The smell of the compound, if any |
| Hardness | The resistance of a solid to being scratched |
| Conductivity | Ability to conduct electricity or heat |
| Feature | Inorganic Compounds | Organic Compounds |
|---|---|---|
| Basic Elements | Mainly contain metals and non-metals, rarely contain carbon | Primarily composed of carbon and hydrogen |
| Carbon-Hydrogen Bonds | Lacks carbon-hydrogen bonds | Always contains carbon-hydrogen bonds |
| Complexity | Generally simpler structures | More complex structures with long chains and rings |
| Melting and Boiling Points | Often have high melting and boiling points | Varies widely, generally lower than inorganic compounds |
| Solubility | Soluble in water, insoluble in organic solvents | Insoluble in water, soluble in organic solvents |
| Conductivity | Often good conductors of electricity when dissolved in water | Poor conductors of electricity |
| Formation | Can form salts | Rarely form salts |
| Occurrence | Found in earth’s crust, oceans, atmosphere | Derived from living organisms or synthetic processes |
Estimating the exact number of inorganic compounds is challenging due to the vast array of elements and the possible combinations among them. In the world of chemistry, new inorganic compounds are being synthesized regularly, and the number continues to grow. Currently, there are thousands of known inorganic compounds. These include simple compounds like water and sodium chloride, as well as more complex ones such as transition metal complexes and inorganic polymers. The diversity and abundance of inorganic compounds are due to the ability of elements to form various types of bonds and structures, from simple ions and salts to complex crystalline lattices.
Yes, water (H₂O) is considered an inorganic compound. This might seem counterintuitive since it is vital for all known forms of life and often associated with organic processes. However, chemically speaking, water does not contain carbon-hydrogen bonds, which are a typical hallmark of organic compounds. Water is composed of two hydrogen atoms bonded to one oxygen atom. It is one of the simplest examples of inorganic compounds, yet it plays critical roles in the environment and biological systems, acting as a solvent and participating in countless chemical reactions.
The transformation from inorganic to organic compounds is a fundamental concept in chemistry, particularly in scenarios like the origin of life and synthetic chemistry. In nature, simple inorganic molecules are often converted into organic substances through biological processes. For example, plants convert carbon dioxide (a simple inorganic compound) into organic molecules like glucose through photosynthesis, a process catalyzed by the sun’s energy.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which of the following is a common use for sodium hydroxide (NaOH)?
Food preservative
Cleaning agent
Fertilizer
Glass production
What is the chemical name of the compound with the formula HCl?
Hydrogen chloride
Hydrochloric acid
Hydrofluoric acid
Hypochlorous acid
What is the primary use of silicon dioxide (SiO2)?
Fertilizer
Glass production
Disinfectant
Baking ingredient
Which of the following is a property of ionic compounds?
Low melting points
High electrical conductivity in solid state
High melting points
Poor solubility in water
What is the common name for CaCO3?
Baking soda
Table salt
Limestone
Gypsum
Which of the following is the primary component of table salt?
NaCl
KCl
CaCl2
MgCl2
What is the chemical name of the compound with the formula KMnO4?
Potassium permanganate
Potassium manganate
Potassium chloride
Potassium carbonate
Which inorganic compound is used in fire extinguishers and soft drinks?
CO2
NH3
H2O
SO2
Which compound is commonly used to neutralize acid in the stomach?
NaOH
Mg(OH)2
HCl
NaHCO3
Which compound is commonly used as a desiccant to absorb moisture?
NaCl
CaCl2
KCl
H2O
Before you leave, take our quick quiz to enhance your learning!

