What does stoichiometry primarily involve?
Measurement of energy
Balancing chemical equations
Calculation of reaction rates
Determination of pH levels

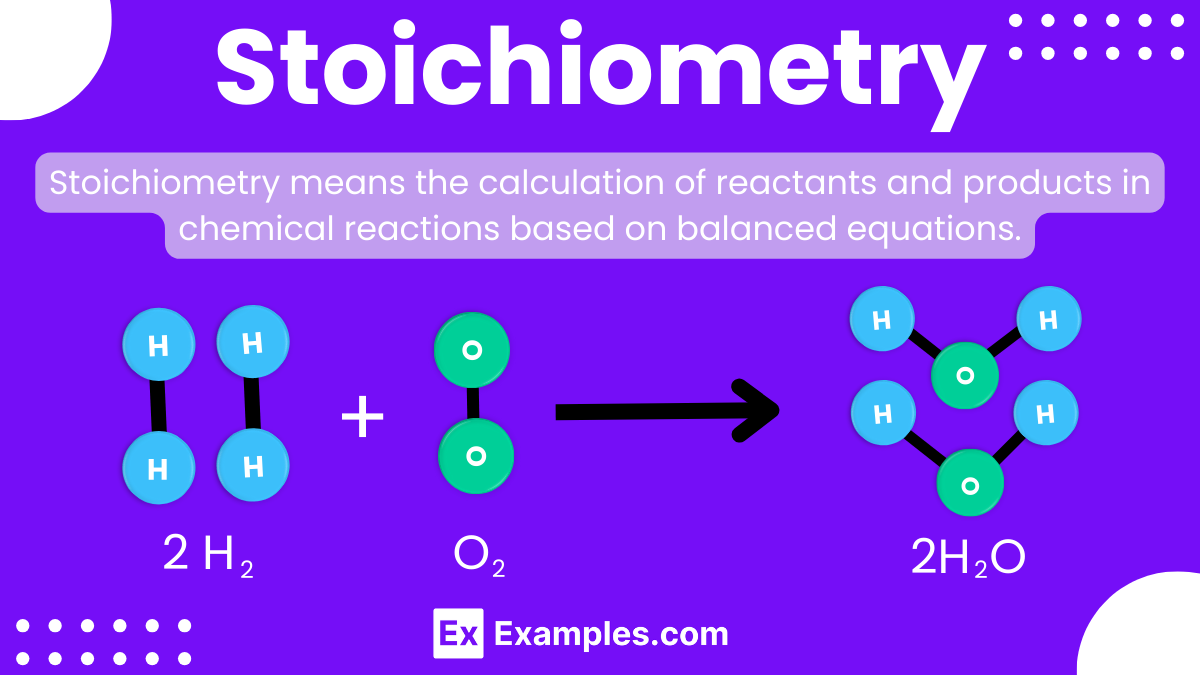
Stoichiometry is a fundamental concept in chemistry that helps us understand the quantitative relationships between the reactants and products in a chemical reaction. By using stoichiometry, we can calculate how much of each substance is needed or produced. It involves using balanced chemical equations to determine the proportions of reactants and products, allowing us to predict the outcomes of reactions accurately. This concept is essential for conducting experiments and industrial processes, as it ensures the correct amounts of chemicals are used, making reactions efficient and safe.
The formula of stoichiometry involves using the balanced chemical equation to determine the relationships between reactants and products in a chemical reaction. By employing the coefficients of the balanced equation, you can calculate the moles of each substance involved. The key stoichiometric relationships include mole-to-mole ratios, mass-to-mass conversions, and volume relationships for gases at standard temperature and pressure (STP). To solve stoichiometry problems, you need to convert quantities of reactants or products into moles, use the balanced equation to find the corresponding number of moles of the desired substance, and then convert back to the required units, whether they are mass, volume, or number of particles.
Balancing chemical equations ensures that the same number of each type of atom is present on both sides of the equation, following the law of conservation of mass. To balance a chemical equation, you start by writing the unbalanced equation with the correct chemical formulas for all reactants and products. Next, identify the number of atoms of each element in both the reactants and the products. Adjust the coefficients (the numbers in front of the molecules) to get the same number of atoms of each element on both sides.
For example, consider the reaction between hydrogen and oxygen to form water:
Unbalanced: 𝐻₂ + 𝑂₂ → 𝐻₂𝑂
Here, there are 2 hydrogen atoms on the left but only 1 oxygen atom, and 2 oxygen atoms on the left but only 1 on the right. To balance, we adjust the coefficients:
Balanced: 2 𝐻₂ + 𝑂₂ → 2 𝐻₂𝑂
Now, there are 4 hydrogen atoms and 2 oxygen atoms on both sides of the equation. This balanced equation accurately represents the conservation of mass in the chemical reaction.
The mole is a fundamental concept in chemistry that allows chemists to count atoms, molecules, or ions by weighing them. One mole of any substance contains Avogadro’s number of particles, which is 6.022×10²³ particles. This number is so large because atoms and molecules are extremely small. The mole provides a bridge between the atomic scale and the macroscopic scale we can measure in the lab.
To use the mole concept, chemists often refer to the molar mass, which is the mass of one mole of a substance, expressed in grams per mole (g/mol). The molar mass of an element is numerically equal to its atomic mass (found on the periodic table), but expressed in grams. For example, the molar mass of carbon is 12 g/mol, meaning one mole of carbon atoms weighs 12 grams. For compounds, the molar mass is the sum of the molar masses of all the atoms in the molecule.
Using the mole concept, chemists can convert between mass, number of particles, and volume (for gases) to solve various chemical problems. This makes it a crucial tool for understanding and performing chemical reactions quantitatively.
The mole ratio is a key concept that describes the proportional relationship between the quantities of reactants and products in a chemical reaction. We derive the mole ratio from the coefficients of the balanced chemical equation. It represent the number of moles of each substance involved in the reaction.
When we balance a chemical equation, the coefficients indicate how many moles of each reactant combine and how many moles of each product form. The mole ratio allows us to translate these coefficients into practical terms, enabling us to calculate how much of one substance will react with a given amount of another substance or how much product will result from specific amounts of reactants.
By using the mole ratio, we ensure that the calculations follow the law of conservation of mass. It states that matter cannot be created or destroyed in a chemical reaction. This ensures that the total number of atoms of each element remains constant throughout the reaction. The mole ratio thus serves as a conversion factor that enables accurate quantitative analysis of chemical reactions.
In a balanced chemical equation, the stoichiometric coefficient indicates the number of moles of each reactant and product involved in the reaction. By using these coefficients, we ensure that the equation obeys the law of conservation of mass, meaning the number of atoms of each element is the same on both sides of the equation. The stoichiometric coefficients help us determine the precise ratios needed for reactants to fully react and form products.
To balance a chemical equation, we adjust the stoichiometric coefficients to ensure that the total number of atoms for each element is equal on both sides. These coefficients are crucial for calculating the amounts of substances consumed and produced in a chemical reaction, allowing us to predict yields and optimize reaction conditions. The coefficients thus play a vital role in the accurate quantification of chemical processes.
Stoichiometry can be easy with practice. It involves using ratios from balanced equations to calculate reactants and products in chemical reactions.
The main rule of stoichiometry is to balance chemical equations, ensuring the number of atoms for each element is equal on both sides of the equation.
Stoichiometry for dummies simplifies the process of using balanced chemical equations to determine the amounts of reactants and products in a reaction.
Start stoichiometry calculations by writing a balanced chemical equation and identifying the known and unknown quantities.
Calculate the mole ratio by comparing the coefficients of reactants and products in a balanced chemical equation.
The first step in solution stoichiometry is to write and balance the chemical equation for the reaction.
To calculate mass in stoichiometry, use the mole ratio from the balanced equation, convert moles to mass using molar mass.
Stoichiometry is essential for predicting the amounts of substances consumed and produced in chemical reactions, enabling efficient use of reactants and minimizing waste.
To balance a chemical equation, ensure the number of atoms of each element is equal on both sides of the equation by adjusting the coefficients.
Mole ratios, derived from the coefficients in a balanced chemical equation, are used to convert between moles of reactants and products.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does stoichiometry primarily involve?
Measurement of energy
Balancing chemical equations
Calculation of reaction rates
Determination of pH levels
How do you determine the limiting reactant in a chemical reaction?
By calculating the highest molar mass
By finding the reactant with the highest initial quantity
By using the stoichiometric coefficients to compare mole ratios
By measuring the rate of reaction
What is the theoretical yield in a chemical reaction?
The amount of product actually obtained
The amount of reactant used
The maximum amount of product that can be formed
The amount of reactant left unreacted
What information is required to perform stoichiometric calculations?
Temperature and pressure
Molar masses and balanced equation
Volume of gases and concentration
Reaction rate and activation energy
What does stoichiometry study in a chemical reaction?
Rate of reaction
Energy changes
Quantities of reactants and products
Reaction mechanism
What is the mole ratio used for in stoichiometry?
To determine the rate of a reaction
To balance chemical equations
To find the activation energy
To convert between moles of different substances
What does the term "theoretical yield" refer to in stoichiometry?
The actual amount of product obtained
The maximum amount of product that can be produced
The amount of reactant used
The amount of product predicted by the reaction rate
What is the purpose of a balanced chemical equation in stoichiometry?
To calculate reaction rates
To determine energy changes
To provide mole ratios for calculations
To identify the reaction mechanism
What is the role of a stoichiometric coefficient in a balanced chemical equation?
To show the reaction rate
To indicate the energy required
To determine the amount of each substance involved
To explain the reaction mechanism
Which factor is not directly considered in stoichiometric calculations?
Temperature of the reaction
Balanced chemical equation
Mole ratios
Amounts of reactants and products
Before you leave, take our quick quiz to enhance your learning!

