What is a molecule?
A single atom
A substance composed of different elements
A group of two or more atoms bonded together
A mixture of various compounds

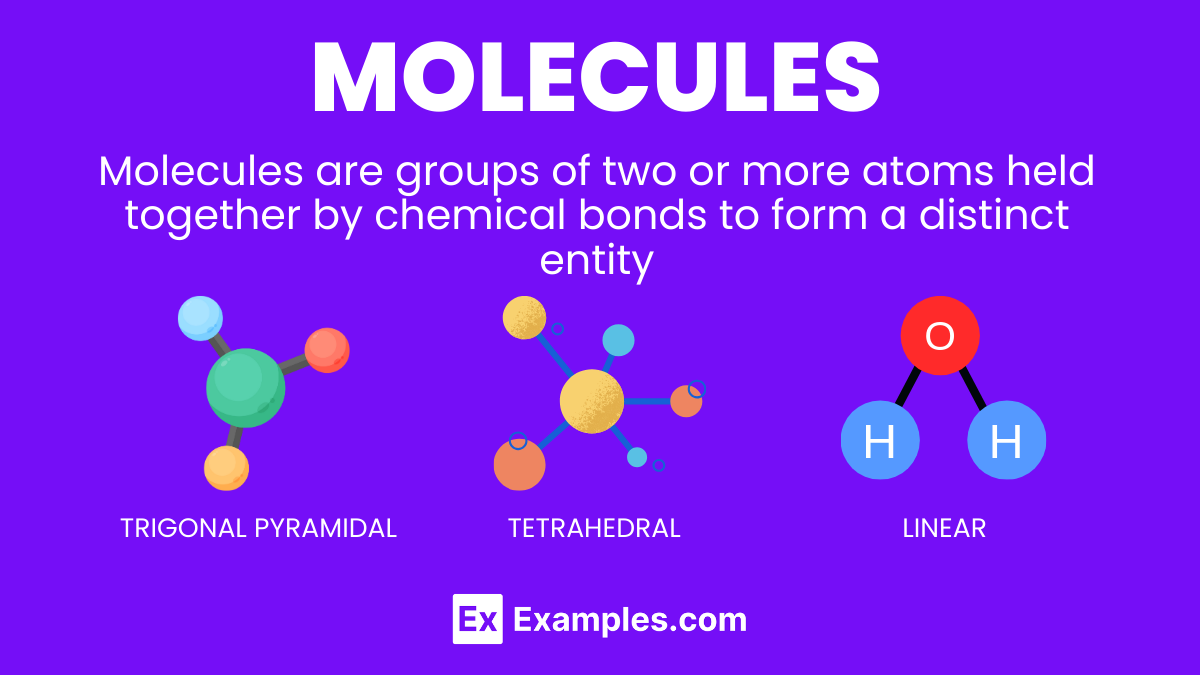
Dive into the world of molecules, the fundamental building blocks of matter that shape everything around us. This comprehensive guide illuminates the intricate dance of atoms bonding to form molecules, essential for life and the universe’s vast complexity. From water’s simple H2O to DNA’s sophisticated spiral, we unravel the mysteries behind molecular formation, properties, and their pivotal roles in chemical reactions. Perfect for students and educators, our examples demystify chemistry’s core concepts, enhancing understanding and sparking curiosity in the microscopic world that constructs our macroscopic reality.
A molecular formula represents the exact number and types of atoms in a molecule using element symbols and numerical subscripts. It indicates the composition of a chemical compound, providing essential information about its structure. By showing the specific elements and their quantities, the molecular formula allows chemists to identify substances, understand their properties, and predict their behavior in reactions.
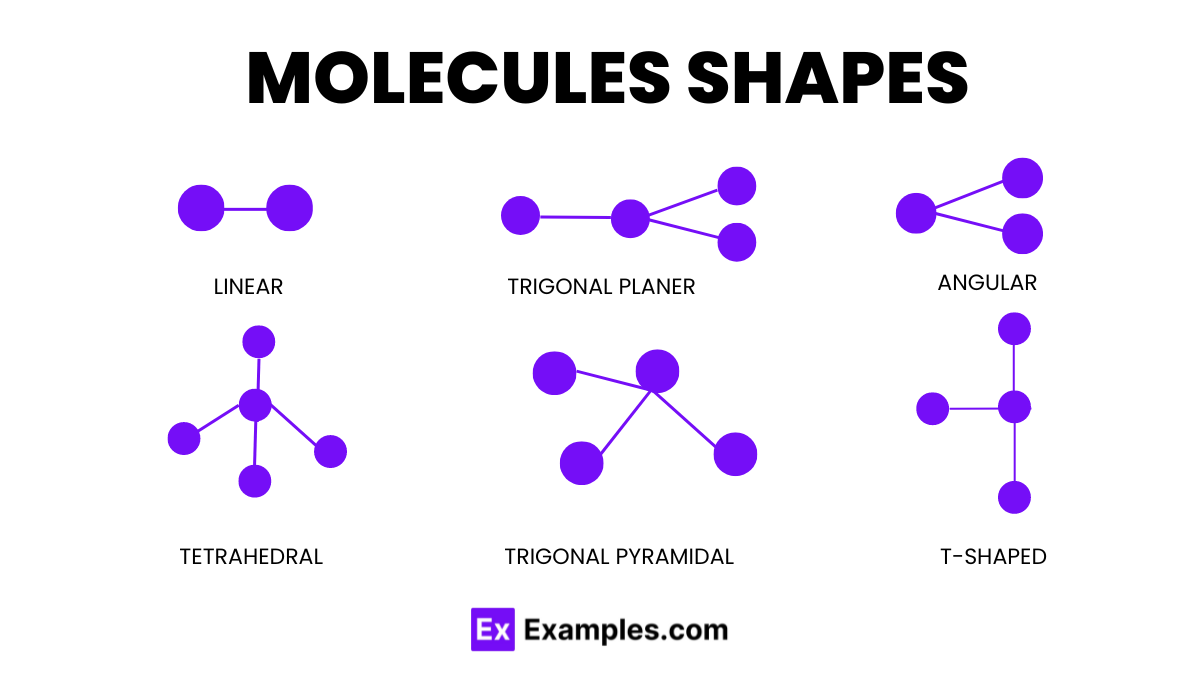
Molecule shapes determine the three-dimensional arrangement of atoms within a molecule, influencing its chemical properties and reactions. The shape of a molecule results from the repulsion between electron pairs around a central atom, described by the Valence Shell Electron Pair Repulsion (VSEPR) theory.
Diatomic molecules consist of two atoms, which can be of the same or different elements. Examples include:
Polyatomic molecules contain more than two atoms. Examples include:
Homonuclear molecules are composed of atoms of the same element. Examples include:
Heteronuclear molecules consist of atoms of different elements. Examples include:
Organic molecules contain carbon atoms and are typically found in living organisms. Examples include:
Inorganic molecules do not primarily contain carbon atoms. Examples include:
Macromolecules are large, complex molecules typically found in biological systems. Examples include:
Molecular bonding is a crucial concept in the realm of chemistry that explains how atoms are held together in molecules. As educators, your role is pivotal in demystifying this complex topic for your students, using simple, understandable English to foster a deep understanding of the subject matter.
In covalent bonds, atoms share electrons to achieve stability. This type of bond often forms between nonmetal atoms. For example, in a water molecule (H₂O), oxygen shares electrons with two hydrogen atoms.
Ionic bonds form when one atom donates an electron to another atom, creating ions that attract each other. This bond usually forms between metal and nonmetal atoms. For instance, in sodium chloride (NaCl), sodium donates an electron to chlorine.
Metallic bonds occur between metal atoms. In these bonds, electrons move freely among a lattice of metal cations, creating a sea of electrons. This bond gives metals their characteristic properties like conductivity and malleability.
Hydrogen bonds form when a hydrogen atom, already bonded to a highly electronegative atom like oxygen or nitrogen, experiences attraction to another electronegative atom. These bonds are weaker than covalent and ionic bonds but are crucial in biological molecules like DNA.
Van der Waals forces include attractions between molecules due to temporary dipoles. These forces are the weakest type of molecular bonding but play a significant role in the physical properties of molecules, like boiling and melting points.
The size of a molecule depends on the number and types of atoms it contains and the arrangement of these atoms. Molecules can vary significantly in size, from small molecules like water (H₂O) with just three atoms, to large complex molecules like proteins, which can contain thousands of atoms.
Molecular size affects a molecule’s physical and chemical properties, including its boiling and melting points, solubility, and reactivity. Scientists measure molecular size using techniques like X-ray crystallography and electron microscopy to understand better how molecules interact and function in different environments.
| Feature | Molecule | Compound |
|---|---|---|
| Definition | The smallest unit of a chemical substance that can exist independently, consisting of one or more atoms bonded together. | A substance made up of atoms of two or more different elements joined by chemical bonds. |
| Composition | Can be composed of one or more atoms of the same element or different elements. | Must consist of atoms of two or more different elements. |
| Example | Oxygen (O2) can be a molecule made up of two oxygen atoms. Hydrogen gas (H2) is another example, with two hydrogen atoms bonded together. | Water (H2O) is a compound consisting of two hydrogen atoms and one oxygen atom. Sodium chloride (NaCl) is another example, made of sodium and chlorine atoms. |
| Types | Homonuclear (same element) and Heteronuclear (different elements) molecules. | No specific types, but can vary based on the chemical composition and the type of chemical bonds (ionic, covalent, metallic, etc.). |
| Properties | The properties of molecules depend on the types and arrangements of atoms within them. | Compounds have properties that are different from their constituent elements. |
| Bonding | Molecules are formed through covalent bonds (sharing of electrons) but can also exist in metals and ionic associations under broader definitions. | Compounds can be formed through ionic (transfer of electrons) and covalent bonds. |
| Representation | Can be represented by molecular formulas showing the number and types of atoms. | Represented by chemical formulas that indicate the ratio of elements present. |
| Feature | Atom | Molecule |
|---|---|---|
| Definition | The smallest unit of an element that retains the properties of that element. | A group of two or more atoms bonded together, representing the smallest fundamental unit of a chemical compound that can participate in a chemical reaction. |
| Composition | Consists of protons, neutrons, and electrons. | Consists of two or more atoms, which can be the same (e.g., O2) or different (e.g., H2O). |
| Existence | Can exist independently. | Cannot exist independently without atoms; it’s a combination of atoms. |
| Properties | Has a unique number of protons that defines the element. | Has properties that are different from its constituent atoms. |
| Formation | Does not form through bonding; it is the basic building block. | Forms through chemical bonds between atoms. |
| Examples | Hydrogen atom (H), Oxygen atom (O). | Water (H2O), Carbon Dioxide (CO2) |
Molecules, the smallest units of chemical compounds that can exist while retaining the chemical properties of the compound, exhibit several distinct characteristics. Understanding these characteristics is fundamental to the study of chemistry and molecular biology. Below, we delve into some of the key features that define molecules:
Molecules are composed of two or more atoms that are chemically bonded together. These atoms can be of the same element, forming a molecule of an element (e.g., O2, N2), or of different elements, forming a compound (e.g., H2O, CO2). The type and number of atoms in a molecule determine its chemical properties and reactivity.
The atoms within molecules are held together by chemical bonds, primarily covalent bonds, where electrons are shared between atoms. Other types of bonds, such as ionic and hydrogen bonds, can also play a role in the structure and properties of molecules. The strength and type of these bonds significantly affect the molecule’s stability, shape, and interactions with other molecules.
Beyond the molecular formula, the structural formula of a molecule shows how the atoms are arranged and bonded within the molecule. This visual representation is crucial for understanding the molecule’s geometry, which influences its chemical behavior and interactions.
The molecular weight (or molecular mass) is the sum of the atomic weights of all the atoms in a molecule. It is a critical parameter for many chemical calculations, including stoichiometry and concentration calculations in solutions.
Molecules have specific physical and chemical properties, including melting point, boiling point, solubility, and reactivity. These properties arise from the types of atoms in the molecule, their arrangement, and the nature of the chemical bonds between them. For instance, the polarity of a molecule affects its solubility in polar or non-polar solvents.
Isomerism is a phenomenon where molecules with the same molecular formula have different structural arrangements of atoms, leading to different properties. Isomers can have vastly different chemical behaviors, showcasing the importance of atomic arrangement within molecules.
Molecules interact with light in various ways, including absorption, reflection, and fluorescence. These interactions depend on the molecule’s structure and composition and are the basis for many spectroscopic techniques used to identify and study molecules.
The reactivity of a molecule is determined by its composition, structure, and the nature of its chemical bonds. Reactivity influences how a molecule participates in chemical reactions, including the types of reactions it can undergo and its role as a reactant or product.
Molecules form when atoms bond through covalent, ionic, or metallic bonds, sharing or transferring electrons.
Molecular shapes affect physical and chemical properties, including reactivity and interactions with other molecules.
Molecular sizes vary based on the number and arrangement of atoms, from small molecules like H₂O to large proteins.
Molecular bonds determine properties like melting/boiling points, solubility, and chemical reactivity.
A molecule is like a tiny building block made of atoms stuck together, creating everything around us, like water and air.
A molecule is a group of two or more atoms bonded together, forming the smallest unit of a chemical compound with unique properties.
The smallest molecule is the hydrogen molecule (H₂), consisting of just two hydrogen atoms bonded together.
The heaviest molecules are often large proteins or synthetic polymers, with some protein molecules reaching millions of atomic mass units (amu).
Yes, H₂O is a molecule composed of two hydrogen atoms and one oxygen atom, commonly known as water.
Polyatomic molecules contain more than two atoms, such as H₂O, CO₂, and C₆H₁₂O₆.
Text prompt
Add Tone
Molecules Shapes
What are the Different Types of Molecules?
What is a molecule?
A single atom
A substance composed of different elements
A group of two or more atoms bonded together
A mixture of various compounds
Which of the following is an example of a diatomic molecule?
CO₂
O₂
H₂O
NaCl
In which state of matter do molecules have the most energy?
Solid
Liquid
Gas
Plasma
What type of bond holds the atoms in a molecule of water together?
Ionic bond
Covalent bond
Hydrogen bond
Metallic bond
Which molecule is known as the universal solvent?
Methanol
Acetone
Water
Benzene
Which of the following is a polar molecule?
CO₂
CH₄
NH₃
O₂
What is the molecular formula of glucose?
C₆H₁₂O₆
C₂H₅OH
CH₃COOH
H₂SO₄
What type of molecule is DNA?
Carbohydrate
Lipid
Protein
Nucleic acid
Which of the following molecules is formed by ionic bonds?
CO₂
NaCl
H₂O
CH₄
Which molecule is essential for cellular respiration?
H₂O
CO₂
O₂
N₂
Before you leave, take our quick quiz to enhance your learning!

