Which method of heat transfer relies on the movement of fluid or gas due to temperature differences?
Conduction
Convection
Radiation
Advection
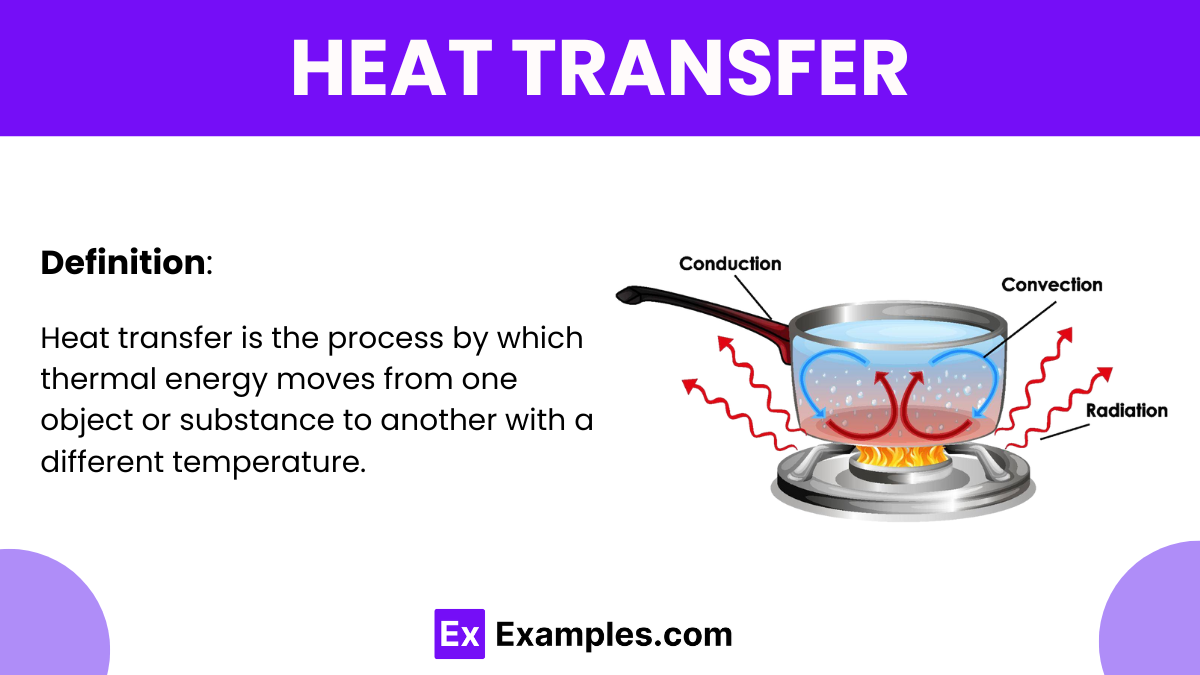
Heat transfer is the process by which thermal energy moves from one object or material to another, driven by temperature differences. It occurs through three primary mechanisms: conduction, where heat moves through a solid material; convection, involving the movement of heat through fluids (liquids or gases); and radiation, which involves heat transfer through electromagnetic waves. This fundamental concept is crucial in understanding how energy flows in both natural environments and engineered systems, affecting everything from weather patterns to the design of buildings and vehicles. Heat transfer plays a key role in the efficiency of heating and cooling systems, as well as in numerous industrial processes.
Heat transfer is the movement of thermal energy from one body or substance to another due to a temperature difference between them. This process is fundamental in various physical phenomena and can occur in three primary ways:
Convection: In this method, heat is transferred through a fluid (which can be a liquid or gas) via the bulk movement of the fluid itself. This happens when the fluid is heated, becomes less dense, and rises, while cooler fluid moves down to replace it, creating a circulation pattern.
Radiation: Heat can also be transferred through electromagnetic waves, such as infrared radiation. This type of heat transfer can occur in a vacuum and does not require a medium; for example, the heat from the sun reaches Earth through radiation.
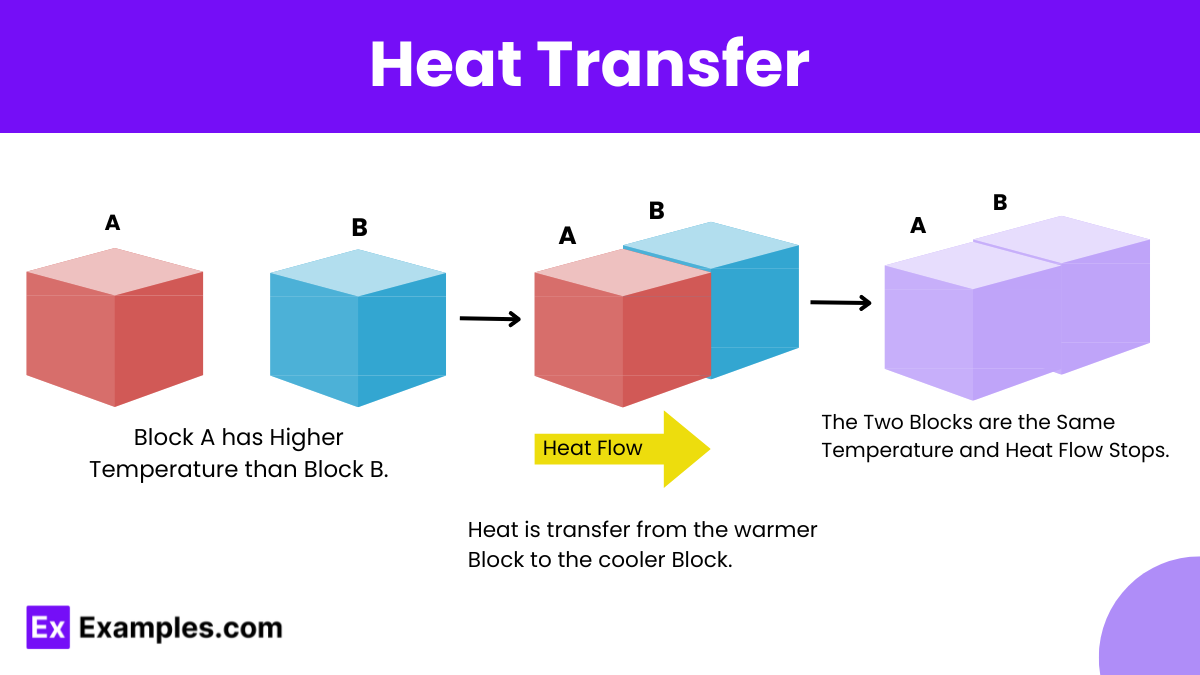
One of the best and most relatable examples of heat transfer is the cooling of a cup of hot coffee left at room temperature. This example beautifully demonstrates two primary modes of heat transfer: convection and conduction.
Additionally, if the room is colder than the coffee, the coffee also loses heat through radiation, emitting infrared radiation into the surrounding cooler environment. However, the conduction through the cup and the convection currents of the air are the most prominent and easily observable processes in this everyday example.
The basic formula for heat transfer by conduction is given by Fourier’s Law:
Where:
Cooking on a Stovetop: When you place a metal pan on a heated stove, the heat from the stove transfers to the pan via conduction. The thermal conductivity (k) of the metal facilitates the flow of heat from the hotter part (the bottom of the pan in contact with the stove) to the cooler parts (the food inside and the air around). The heat transfer rate (Q) can be calculated if you know the thermal conductivity of the pan, the surface area in contact with the heat source (A), the temperature difference between the stove and the bottom of the pan (ΔT), and the thickness of the pan’s bottom (Δx).
The heat transfer by convection can be described by Newton’s Law of Cooling:
Where:
Heating a Room with a Radiator: A radiator heats a room primarily through convection. As the radiator warms the air around it, that warm air expands, becomes less dense, and rises. Cooler air moves in to replace it, getting heated in turn. The heat transfer rate (Q) can be calculated using the convective heat transfer coefficient (h), the surface area of the radiator (A), and the temperature difference between the radiator’s surface and the air (Tₛ−Tբ).
Radiation heat transfer can be calculated using the Stefan-Boltzmann Law:
Earth Receiving Heat from the Sun: The Earth receives heat from the sun through radiation across the vacuum of space. The heat transfer (Q) due to radiation can be calculated using the Stefan-Boltzmann Law. Here, ϵ would be the emissivity of the Earth, σ is the Stefan-Boltzmann constant, A is the cross-sectional area of the Earth facing the sun, Ts is the surface temperature of the sun, and 𝑇𝑠𝑢𝑟𝑟𝑜𝑢𝑛𝑑𝑖𝑛𝑔𝑠 is the temperature of outer space around the Earth.
| Quantity | Symbol | Unit | Description |
|---|---|---|---|
| Heat Transfer Rate | Q | Watts (W) | Measures the amount of heat transferred per unit time. |
| Thermal Conductivity | k | Watts per meter-kelvin (W/m·K) | Measures a material’s ability to conduct heat. |
| Convective Heat Transfer Coefficient | h | Watts per square meter-kelvin (W/m²·K) | Measures the effectiveness of convection. |
| Stefan-Boltzmann Constant | σ | Watts per square meter per kelvin to the fourth (W/m²·K⁴) | Fundamental constant in the equation for radiative heat transfer. |
| Temperature Difference | ΔT | Kelvin (K) or degrees Celsius (°C) | Used to calculate the driving force for heat transfer. |
| Emissivity | ϵ | Dimensionless | Measures the effectiveness of a surface in emitting energy as radiation. |
Heat transfer is a fundamental concept within the field of thermodynamics, which deals with the movement of heat between systems and their surroundings and forms an essential aspect of the study of energy transformations. Here’s how heat transfer fits into the broader scope of thermodynamics:
This law states that energy cannot be created or destroyed, only transformed from one form to another. In terms of heat transfer, this means that the heat energy lost by one body is gained by another. The heat transfer across a system boundary is accounted for in the energy balance of the system.
This law states that the entropy of the universe, or of an isolated system, always increases over time. Heat transfer is often accompanied by changes in entropy. Specifically, spontaneous heat transfer (without external work) occurs from higher temperature to lower temperature, thereby increasing entropy.
Heat transfer is fundamental in appliances like refrigerators, ovens, and air conditioners. These devices rely on efficient heat transfer to cool or heat spaces, preserve food, and cook meals.
Yes, heat transfer can occur in a vacuum through radiation. Unlike conduction and convection, which require a medium, radiation involves electromagnetic waves that can travel through empty space, as seen with sunlight reaching Earth.
Materials with high thermal conductivity, such as copper and aluminum, are best for heat transfer. These materials facilitate the rapid movement of heat, making them ideal for cooking utensils, radiators, and electronic heat sinks.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which method of heat transfer relies on the movement of fluid or gas due to temperature differences?
Conduction
Convection
Radiation
Advection
Heat transfer by radiation occurs primarily through which of the following?
Direct contact between materials
Convection currents in fluids
Electromagnetic waves
Thermal conductivity of solids
The transfer of heat through a solid material without the movement of the material itself is known as:
Conduction
Convection
Radiation
Induction
In a vacuum, heat transfer between objects occurs primarily by:
Conduction
Convection
Radiation
Advection
Which material would typically have the highest thermal conductivity?
Plastic
Wood
Aluminum
Styrofoam
Insulating materials work primarily by:
Increasing thermal conductivity
Reducing convective heat transfer
Enhancing radiation absorption
Increasing molecular motion
The heat absorbed or released by a substance during a change in state is called:
Latent heat
Specific heat
Thermal capacity
Enthalpy
Which of the following is NOT a method to reduce heat loss in buildings?
Double-glazed windows
Insulating walls
Painting walls black
Draft-proofing doors and windows
The rate of heat transfer through a material is influenced by all of the following EXCEPT:
Material thickness
Temperature difference
Material color
Surface area
Which statement best describes how a thermos flask reduces heat transfer?
By increasing conduction
By reducing convection and radiation
By enhancing convection
By reducing thermal conductivity
Before you leave, take our quick quiz to enhance your learning!

