What is the formula for calculating latent heat?
Q = mcΔT
Q = m/L
Q = mL
Q = L/m

Latent heat is a critical concept in physics, particularly when studying the changes in the state of matter, such as melting or boiling. The latent heat formula helps calculate the amount of energy absorbed or released during these phase changes without a change in temperature. This formula is especially useful in understanding how much energy substances require to change from solid to liquid or from liquid to gas and vice versa.
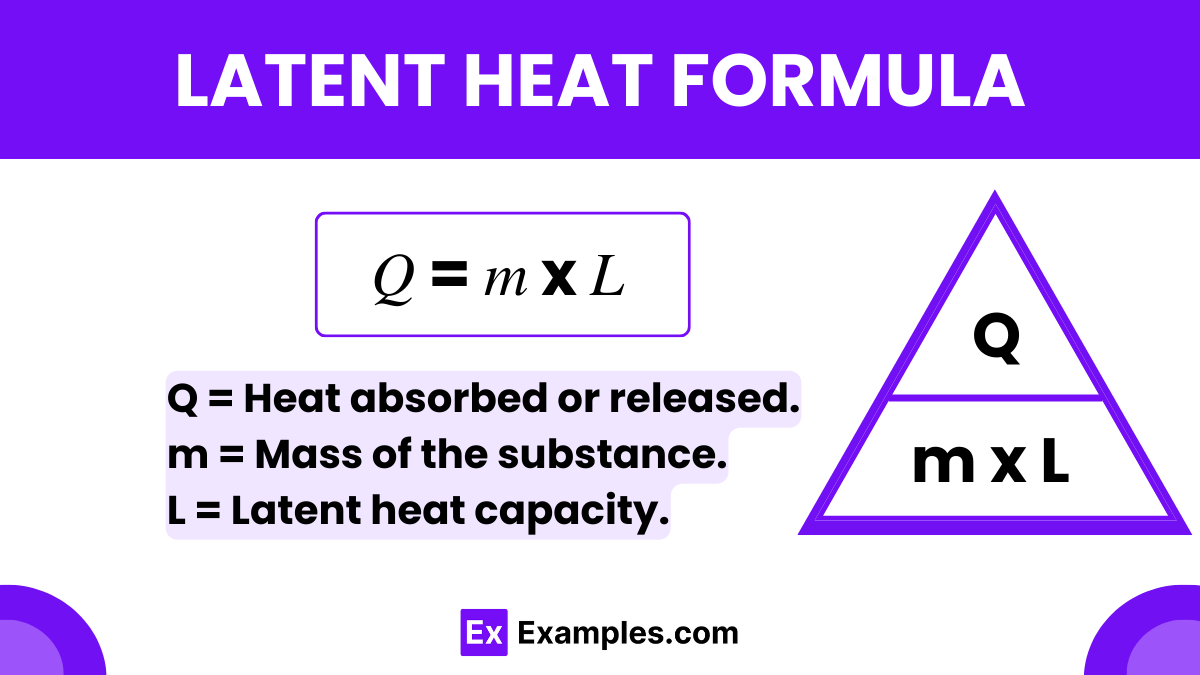
The latent heat formula is expressed as
L = Q / m
The concept of latent heat was first introduced by Joseph Black, a Scottish physicist, in the 18th century. He made significant contributions to thermodynamics, distinguishing between heat that changes temperature and heat that changes physical state.
To derive the latent heat formula, let’s begin with the understanding that during a phase change, such as ice melting into water or water vaporizing into steam, the temperature of the substance does not change. Instead, the energy provided goes into changing the state of the substance. This energy change is what we refer to as latent heat.
First, consider the relationship between energy and mass in the context of phase changes. The amount of energy required to change the phase of a substance depends on how much of the substance you have. This means that the energy involved in the phase change is directly proportional to the mass of the substance. We express this relationship as:
Where:
Next, we introduce a proportionality constant, L is known as the latent heat capacity. This constant represents the amount of energy required to change the phase of one kilogram of a substance without changing its temperature.
By replacing the proportionality with an equality, we get the latent heat formula:
Where:
Thus, the latent heat formula 𝑄=𝑚L effectively quantifies how much energy is absorbed or released when a specific mass of a substance undergoes a phase change. This formula is essential in physics for calculating energy transformations in processes like melting, boiling, or condensing.
Question: How much energy is required to melt 500 grams of ice at 0°C? The latent heat of fusion for ice is 334,000 joules/kg.
Solution:
Identify the given values:
Mass, 𝑚 = 500 grams = 0.5 kg (since 1 kg = 1000 grams)
Latent heat of fusion, 𝐿 = 334,000 joules/kg
Apply the latent heat formula:𝑄=𝑚 x 𝐿
Q=mL
Q = 0.5 kg × 334,000 J/kg
𝑄=167,000 joules
Explanation: To melt 0.5 kg of ice, 167,000 joules of energy are needed. This energy goes into breaking the molecular bonds in the ice without raising its temperature.
Question: Calculate the amount of energy released when 2 kg of steam at 100°C condenses into water at the same temperature. The latent heat of vaporization of water is 2,260,000 joules/kg.
Solution:
Identify the given values:
Mass, 𝑚 = 2 kg
Latent heat of vaporization, 𝐿 = 2,260,000 joules/kg
Apply the latent heat formula:
𝑄=𝑚𝐿
𝑄 = 2 kg × 2,260,000 J/kg
𝑄 = 4,520,000 joules
Explanation: When 2 kg of steam condenses, it releases 4,520,000 joules of energy. This released energy can significantly warm the surroundings, as the steam transfers its hidden heat to the environment.
L represents the latent heat capacity, measuring energy per unit mass required for a phase change, expressed in joules per kilogram (J/kg).
Multiply the mass of the substance by its latent heat of vaporization value: 𝑄=𝑚𝐿, where 𝐿 is specific to vaporization.
Use the formula 𝑄=𝑚𝐿, substituting 𝐿 with the latent heat of fusion specific to ice to determine the required energy.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the formula for calculating latent heat?
Q = mcΔT
Q = m/L
Q = mL
Q = L/m
What does the symbol L represent in the latent heat formula?
Specific heat
Latent heat
Heat capacity
Thermal conductivity
Which of the following best defines latent heat?
The heat required to raise the temperature of 1 kg of a substance by 1°C
The heat required to change the phase of 1 kg of a substance without changing its temperature
The total heat content of a substance
The heat lost by a substance during cooling
Which of the following phases changes involves latent heat of fusion?
Ice to water
Water to steam
Steam to water
Water to ice
Which phase change requires the latent heat of vaporization?
Solid to liquid
Liquid to gas
Gas to liquid
Liquid to solid
Which phase change involves the latent heat of sublimation?
Solid to liquid
Liquid to gas
Solid to gas
Liquid to solid
What is the heat required to convert 2 kg of water at 100°C to steam, given the latent heat of vaporization is 2260 kJ/kg?
1130 kJ
2260 kJ
4520 kJ
6780 kJ
Which of the following statements correctly describes latent heat?
Latent heat is the heat required to change the temperature of a substance.
Latent heat is the heat required to change the phase of a substance without changing its temperature.
Latent heat is the heat lost during cooling.
Latent heat is the heat generated during chemical reactions.
What happens to the temperature of a substance during the phase change when latent heat is absorbed?
The temperature increases.
The temperature decreases.
The temperature remains constant.
The temperature fluctuates.
Which phase change involves the latent heat of vaporization?
Freezing
Melting
Boiling
Sublimation
Before you leave, take our quick quiz to enhance your learning!

