What is the freezing point of water in Kelvin?
0 K
100 K
273 K
373 K
The Kelvin scale is a fundamental temperature scale in physics, named after Lord Kelvin. It starts at absolute zero, the theoretical point where all molecular motion ceases, marked as 0 Kelvin (K). Unlike Celsius and Fahrenheit, Kelvin does not use degrees and directly correlates with Celsius for scientific accuracy: 0°C equals 273.15 K. This scale is essential in fields ranging from theoretical physics to practical applications in various technologies, providing a precise measurement system that is crucial for scientific calculations across the globe.
The Kelvin scale is directly related to the Celsius scale with a simple formula to convert temperatures between them. Here’s the formula for converting Celsius to Kelvin:
Where:
This formula shows that Kelvin and Celsius scales are offset by 273.15 degrees. This means that 0 degrees Celsius, which is the freezing point of water, corresponds to 273.15 Kelvin. Absolute zero, the theoretical minimum temperature, is 0 Kelvin, which equals -273.15 degrees Celsius.
Converting a temperature from Celsius to Kelvin is straightforward. Here’s the simple step-by-step process:
Here’s the formula you use for the conversion:
𝐾 = 𝐶+273.15
If you have a temperature of 25°C and want to convert it to Kelvin: 𝐾=25+273.15=298.15 K
Now, you have converted 25°C to Kelvin, which is 298.15 K. This method is used in all scientific contexts where Kelvin is the preferred unit of temperature measurement.
| Aspect | Celsius (°C) | Kelvin (K) |
|---|---|---|
| Unit Name | Named after Anders Celsius | Named after Lord Kelvin |
| Zero Point | Represents the freezing point of water | Represents absolute zero |
| Scale Type | Relative temperature scale | Absolute temperature scale |
| Unit Increment | Each increment is equal to 1°C | Each increment is equal to 1K |
| Common Usage | Widely used in daily life and weather reporting globally | Predominantly used in scientific and engineering contexts |
| Base Reference for Zero | 0°C is the freezing point of water | 0 K is the lowest possible temperature, where molecular motion stops |
| Conversion Formula | 𝐶 = 𝐾−273.15 | 𝐾 = 𝐶+273.15 |
Absolute zero is the theoretical lowest temperature possible, where all molecular motion ceases. In the Kelvin temperature scale, absolute zero is defined as exactly 0 Kelvin (0 K). This temperature corresponds to -273.15 degrees Celsius and -459.67 degrees Fahrenheit. Absolute zero is a cornerstone in the fields of physics and chemistry, particularly in understanding the behavior of gases and the laws of thermodynamics.
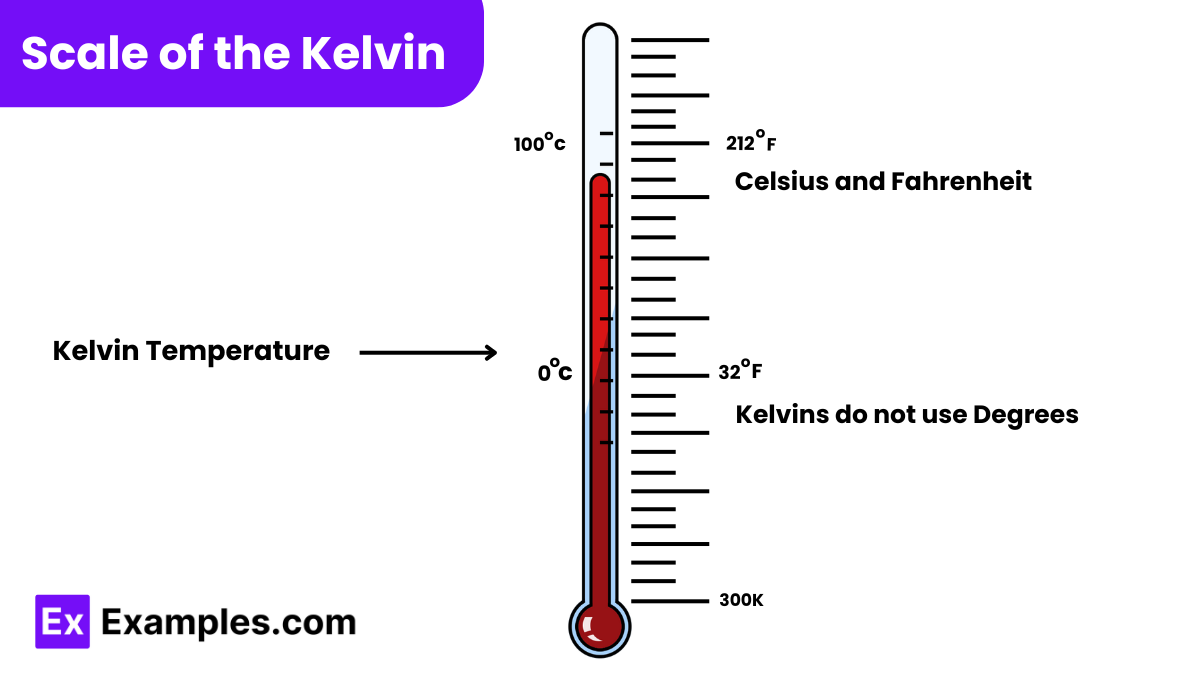
The Kelvin scale is an absolute temperature scale used primarily in the scientific community worldwide. Here are some key characteristics and the scale points associated with it:
Absolute Zero: 0 Kelvin (K) – This is the starting point of the Kelvin scale, representing the lowest possible temperature where all thermal motion of atoms theoretically ceases.
Triple Point of Water: 273.16 K – This is a unique temperature where water can exist simultaneously in gas, liquid, and solid states. This specific point is used to define the Kelvin scale.
Increment: The scale increments by 1 Kelvin, which is equivalent to 1 degree Celsius in magnitude. This means that the temperature interval or thermal unit size is the same as that of the Celsius scale, but the Kelvin scale starts at absolute zero.
Usage: Kelvin is used extensively in scientific measurements because it allows for precise calculations and descriptions of thermodynamic processes, especially in physics and chemistry.
Common Benchmarks: Some common reference temperatures in the Kelvin scale include:
Relative Comparison to Other Scales: Kelvin directly relates to the Celsius scale by a simple addition or subtraction of 273.15, making it easy to convert between the two. Unlike Fahrenheit, which requires a more complex conversion formula, Kelvin and Celsius share the same incremental scale, differing only in their starting point (zero value).
The Kelvin scale is critical for scientific experiments and calculations, as it provides a thermodynamically absolute frame of reference that simplifies many formulae and theoretical models.
Learning and Teaching: The introduction of Kelvin in education facilitates the understanding of absolute temperatures and basic physical concepts, such as entropy and the zeroth law of thermodynamics.
The triple point of water is the temperature and pressure at which water can coexist in three states: solid, liquid, and gas. It is precisely defined as 273.16 K. This point is used to calibrate temperature measurements and define the Kelvin scale with high precision.
Kelvin is used as an absolute scale for scientific accuracy, so it measures temperature in Kelvins, not degrees. This emphasizes that the scale starts from an absolute zero, which is different from relative temperature scales like Celsius and Fahrenheit.
Absolute zero (0 K) is the theoretical lowest possible temperature, where all thermal motion ceases. This concept is crucial for understanding the fundamental principles of thermodynamics and quantum mechanics.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the freezing point of water in Kelvin?
0 K
100 K
273 K
373 K
What is the boiling point of water in Kelvin?
100 K
273 K
373 K
473 K
If the temperature is 25°C, what is the temperature in Kelvin?
248 K
273 K
298 K
323 K
Which of the following is the absolute zero temperature?
0°C
0°F
0 K
-273 K
A temperature of 300 K is equivalent to how many degrees Celsius?
27°C
37°C
47°C
57°C
What is the typical room temperature in Kelvin?
273 K
293 K
298 K
310 K
If the temperature of an object increases by 10°C, by how much does the temperature increase in Kelvin?
10 K
20 K
30 K
40 K
Which of the following temperatures is the highest?
0°C
0°F
0 K
100 K
Which of the following is NOT a valid Kelvin temperature?
-1 K
0 K
273 K
373 K
If a scientist measures a temperature of 77 K, what is this temperature in Celsius?
-196°C
-173°C
-123°C
-96°C
Before you leave, take our quick quiz to enhance your learning!

