What is the atomic number of Tellurium?
50
52
54
56
Dive into the world of Tellurium with our detailed guide. This often-overlooked element holds a unique place in the periodic table. As educators, understanding Tellurium’s properties and applications can enrich science lessons and spark students’ curiosity. Our guide covers everything from its fundamental definition to its practical uses, providing valuable insights for classroom discussions. Embrace the opportunity to enhance your teaching toolkit with knowledge about Tellurium.
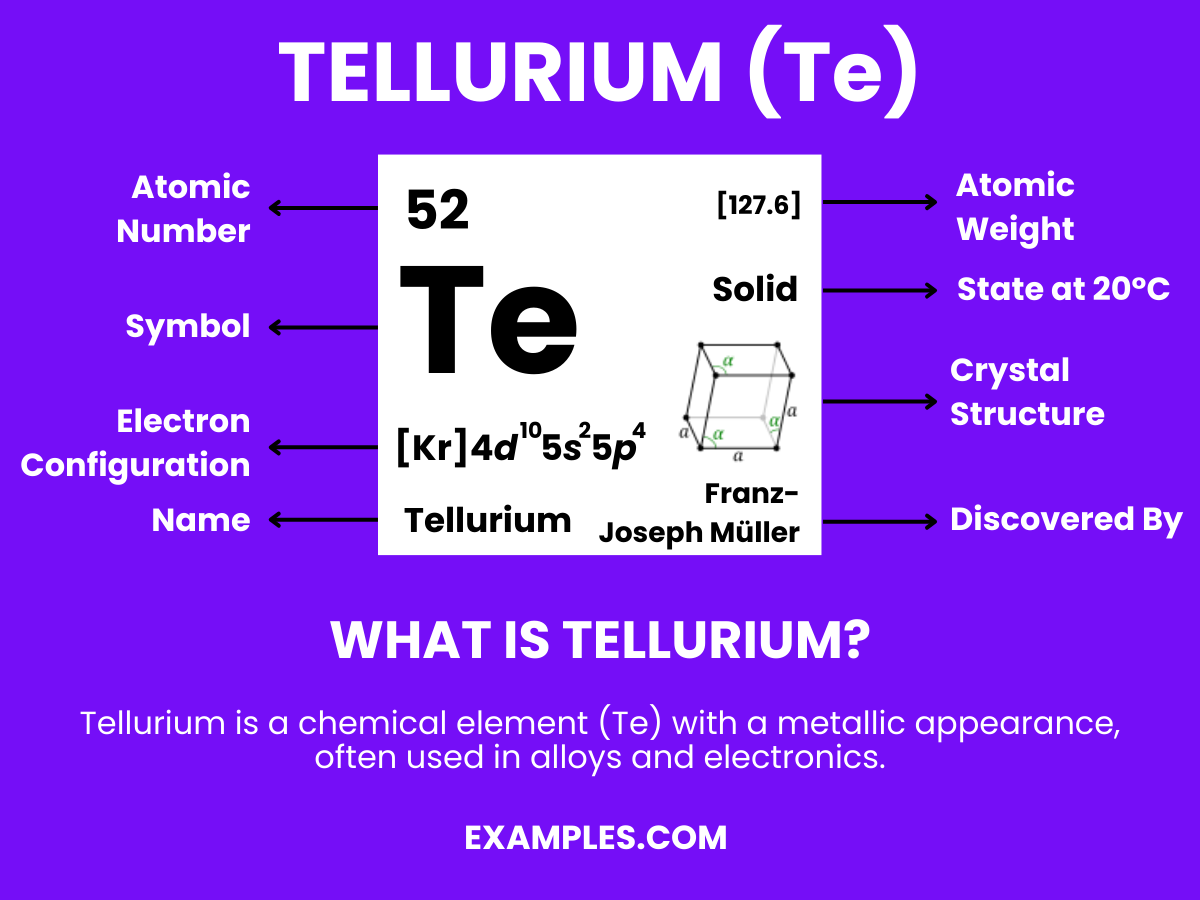
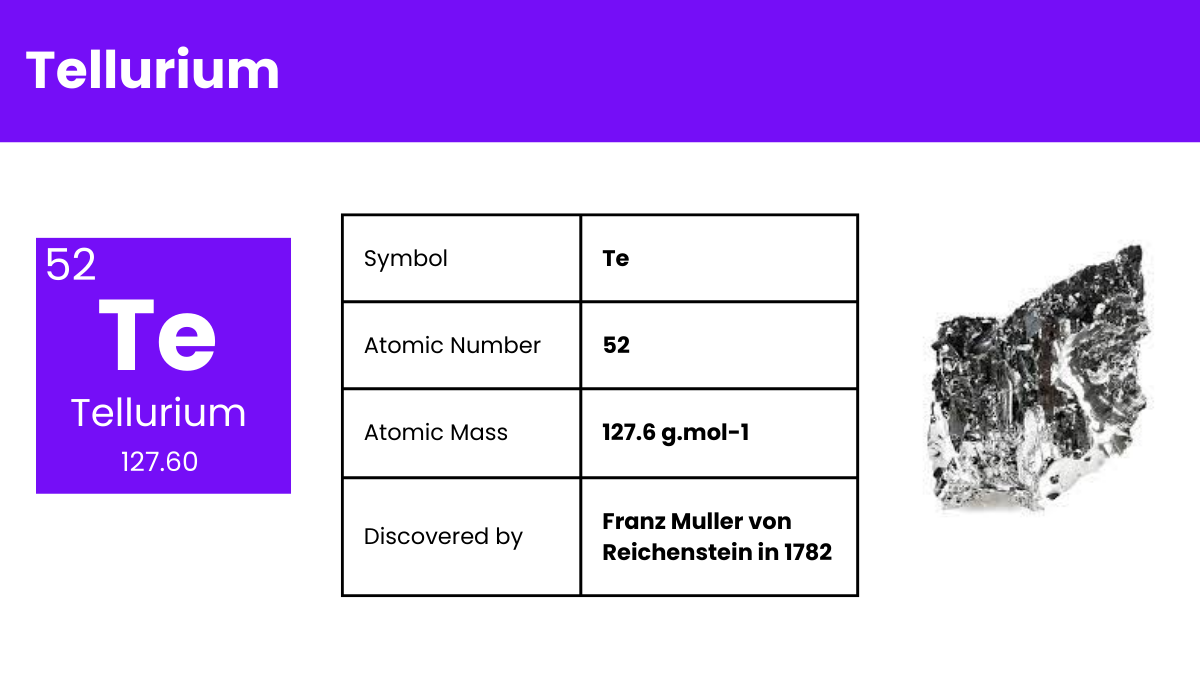
Tellurium is a chemical element with a distinct identity in the periodic table. It’s a relatively rare, silver-white, brittle metalloid. Often used in alloys and electronics, Tellurium has properties that are intermediate between metals and nonmetals, making it a fascinating subject for scientific study. Its diverse applications range from solar panels to thermoelectric devices. Understanding Tellurium not only enriches chemistry knowledge but also bridges the gap between theoretical science and real-world applications, making it a compelling topic for educators to explore with their students.
| Boron (B) |
| Silicon (Si) |
| Germanium (Ge) |
| Arsenic (As) |
| Antimony (Sb) |
| Property | Detail |
|---|---|
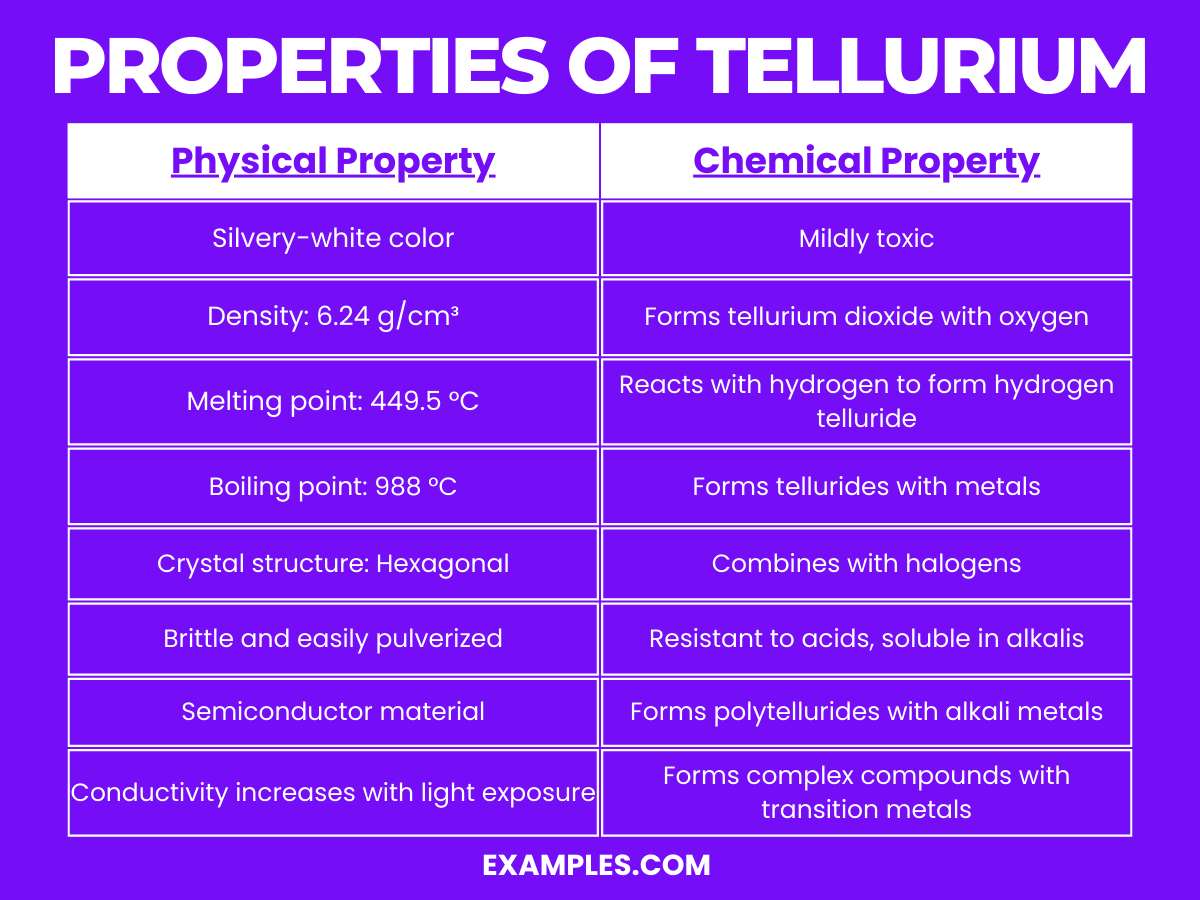
| Appearance | Tellurium has a silvery-white, metallic luster. |
| State at Room Temperature | Solid under standard conditions. |
| Density | 6.24 g/cm³, making it relatively dense. |
| Melting Point | Melts at 449.5 °C, indicating moderate thermal stability. |
| Boiling Point | Boils at 988 °C, demonstrating its capacity to withstand heat. |
| Crystal Structure | Hexagonal crystalline structure, contributing to its brittleness. |
| Conductivity | It is a semiconductor; its conductivity increases with light exposure. |
| Hardness | Relatively brittle and easily pulverized. |
Tellurium’s chemical properties are intriguing and diverse, contributing to its use in various applications:
| Property | Value with Unit |
|---|---|
| Boiling Point | 988 °C |
| Melting Point | 449.51 °C |
| Critical Temperature | Not Available |
| Critical Pressure | Not Available |
| Heat of Vaporization | 52.55 kJ/mol |
| Heat of Fusion | 17.49 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.202 J/g·K |
| Thermal Conductivity | 2.35 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 20°C) | 6.232 kg/m³ |
| Viscosity | Not Applicable (Solid) |
| Solubility in Water | Insoluble |
| Color | Silvery-white |
| Phase at Room Temperature | Solid |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (at 20°C) | 1 × 10^5 µΩ·m |
| Thermal Conductivity | 2.35 W/m·K |
| Electronegativity (Pauling scale) | 2.1 |
| Ionization Energy | 9.0096 eV |
| Electron Affinity | 1.97087 eV |
| Property | Value with Unit |
|---|---|
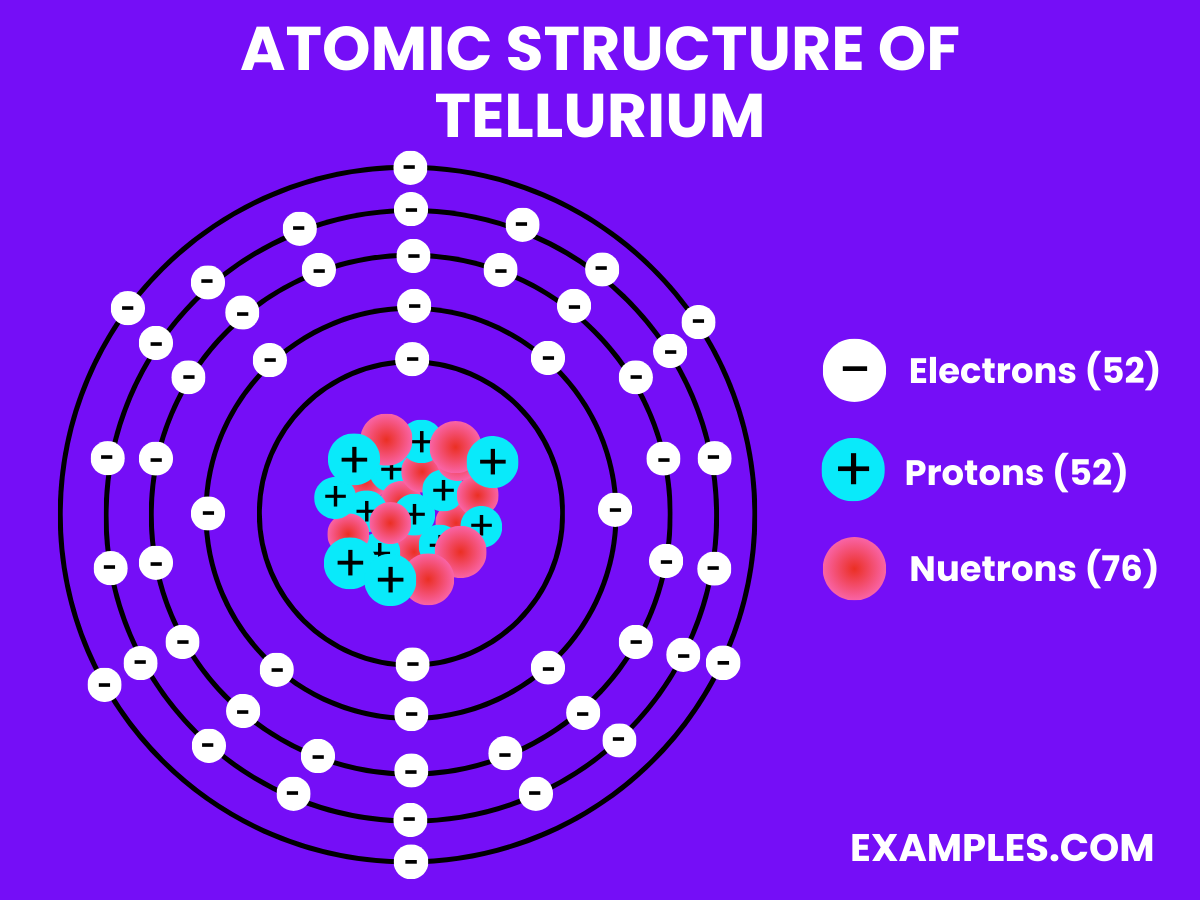
| Atomic Number | 52 |
| Atomic Mass | 127.60 amu |
| Isotopes | ^120Te, ^122Te, ^123Te, ^124Te, ^125Te, ^126Te, ^128Te, ^130Te |
| Nuclear Spin (varies by isotope) | Varies by isotope |
| Neutron Cross Section (varies by isotope) | Varies by isotope |
| Nuclear Magnetic Moment (varies by isotope) | Varies by isotope |
Tellurium, a relatively rare element, forms various chemical compounds with distinct properties and applications. Here, we explore the top six tellurium compounds.
Tellurium dioxide is a solid white powder that exhibits piezoelectric properties, making it useful in acousto-optic devices. It’s used in the manufacture of glass with high refractive indices.
Chemical Equation: Te+O₂→TeO₂
This yellow to white crystalline solid is used as a precursor to other tellurium compounds. It’s employed in the synthesis of organic tellurium compounds in chemistry.
Chemical Equation: Te+2Cl₂→TeCl₄
A colorless, toxic gas, tellurium hexafluoride is notable for its high electron affinity and reactivity towards glass. It is used in semiconductor research.
Chemical Equation: Te+3F₂→TeF₆
An important semiconductor material, cadmium telluride is primarily used in solar cells. It’s known for its efficiency in converting sunlight into electrical power.
Chemical Equation: Cd+Te→CdTe
This compound is a thermoelectric material, which means it can convert temperature differences into electricity, and vice versa. It’s used in cooling and power generation applications.
Chemical Equation: 2Bi+3Te→Bi₂Te₃
Lead telluride is another thermoelectric material known for its effectiveness at high temperatures. It finds its applications in thermoelectric generators and cooling devices.
Chemical Equation: Pb+Te→PbTe
Tellurium has several isotopes, each with unique properties. The table below provides a concise overview of its key isotopes.
| Isotope | Atomic Mass | Natural Abundance (%) | Half-life | Decay Mode |
|---|---|---|---|---|
| Te-120 | 119.90402 | 0.09 | Stable | – |
| Te-122 | 121.90304 | 2.55 | Stable | – |
| Te-123 | 122.90427 | 0.89 | Stable | – |
| Te-124 | 123.90282 | 4.74 | Stable | – |
| Te-125 | 124.90443 | 7.07 | Stable | – |
| Te-126 | 125.90331 | 18.84 | Stable | – |
Tellurium serves as a potent alloying agent, significantly enhancing the machinability of copper and stainless steel. When added to these metals, it forms an alloy that exhibits improved thermal conductivity and a lower melting point. This modification is crucial in creating more malleable and workable metals for industrial applications. The resulting alloy displays enhanced strength and corrosion resistance, making it ideal for use in electronics, machining, and other high-stress environments.
Tellurium is integral in the semiconductor industry. It’s used to create cadmium telluride (CdTe), a vital component in thin-film solar cells. These cells are more cost-effective and environmentally friendly compared to traditional silicon-based cells. Additionally, Tellurium is used in thermoelectric devices which convert heat directly into electricity. This application is particularly valuable in power generation and recycling waste heat into usable energy.
Tellurium imparts a unique color to glass and ceramics. When added in small quantities, it can produce a range of colors from light blue to deep red, depending on the concentration and the other elements present. This property is exploited in the manufacture of colored glass for decorative and architectural purposes. In ceramics, Tellurium enhances thermal resistance, making the material suitable for high-temperature applications.
In rubber production, Tellurium acts as an accelerator in the vulcanization process. This process is essential for enhancing the durability, elasticity, and heat resistance of rubber. The presence of Tellurium in rubber compounds results in products with superior aging properties and increased resistance to abrasion, making it ideal for automotive tires and industrial rubber products.
Tellurium is used as a catalyst in certain chemical reactions, particularly in the refining of crude oil. It helps in the oxidative cracking process, making the breakdown of heavy crude components more efficient. This leads to the production of high-quality fuels and lubricants. Its catalytic properties are also employed in the synthesis of organic compounds, playing a significant role in the pharmaceutical and agrochemical industries.
Tellurium is primarily obtained as a byproduct of copper and lead refining. During the electrorefining of copper, anode slimes form, which contain various valuable elements, including Tellurium. The process involves leaching the anode slimes with sulfuric acid, followed by a series of purification steps to isolate Tellurium.
As environmental and sustainability concerns grow, recovering Tellurium from recycled materials has gained importance. Electronic waste, particularly from older photovoltaic cells and thermoelectric devices, serves as a significant source. The recycling process typically involves chemical treatment to dissolve the Tellurium, followed by precipitation or electro-winning to recover the metal.
Post-extraction, Tellurium undergoes a refining process to achieve the desired purity level. This process may include roasting the Tellurium compounds in air, followed by reduction with carbon or hydrogen. The resulting Tellurium is then purified through processes like zone refining, where impurities are segregated and removed, ensuring a high-purity final product.
Ensuring the quality of Tellurium is crucial for its various applications, especially in electronics and solar energy sectors. The quality control process involves rigorous testing for purity and impurities. Advanced techniques like mass spectrometry and X-ray fluorescence are employed to certify the quality of the Tellurium produced.
The production of Tellurium must adhere to environmental and safety regulations. Handling Tellurium requires precautions due to its toxicity in certain forms. The industry must also address environmental concerns related to mining and refining processes, ensuring minimal ecological impact and compliance with environmental protection standards.
Tellurium is primarily used in alloys, solar panels, thermoelectric devices, colored glass, and rubber production, enhancing durability and efficiency.
Tellurium exposure can cause respiratory irritation, garlic-like body odor, skin rashes, and, in severe cases, liver and kidney damage.
Yes, Tellurium can be harmful; inhaling its dust or vapors leads to respiratory issues, skin irritation, and potential organ damage with prolonged exposure.
Tellurium plays a pivotal role in various industries, from enhancing alloys to revolutionizing solar energy. Despite its benefits, caution is advised due to its potential health hazards. Understanding Tellurium’s uses and impacts is crucial in leveraging its properties while ensuring safety and environmental sustainability. This guide offers comprehensive insights into Tellurium, aiding in informed and responsible utilization.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Tellurium?
50
52
54
56
Tellurium is commonly found in which type of ore?
Bauxite
Chalcopyrite
Galena
Sphalerite
Which property of Tellurium makes it useful in thermoelectric devices?
Magnetic properties
Optical properties
Semiconductor properties
Solubility in water
Tellurium belongs to which group of the periodic table?
Chalcogens
Halogens
Noble gases
Transition metals
Which of the following is a common use of Tellurium in industry?
As a pigment in paints
In vulcanization of rubber
As a catalyst in petroleum refining
As a hardening agent in steel
What is the effect of Tellurium exposure on human health?
Increased blood pressure
Respiratory issues
Skin irritation
Neurological damage
The abundance of Tellurium in the Earth's crust is considered:
Common
Rare
Moderate
Plentiful
Tellurium dioxide is used primarily in:
Glass manufacturing
Food additives
Water treatment
Fertilizers
Which form of Tellurium is toxic to humans?
Metallic Tellurium
Tellurium compounds
Both metallic Tellurium and Tellurium compounds
Neither metallic Tellurium nor Tellurium compounds
Tellurium is primarily produced as a byproduct of the refining of which metal?
Copper
Iron
Zinc
Gold
Before you leave, take our quick quiz to enhance your learning!

