What is the primary use of zinc sulfate in medicine?
To treat hypertension
To supplement zinc deficiency
To reduce cholesterol levels
To treat bacterial infections


Zinc sulfate is a white crystalline salt that plays an important role in chemistry, especially in the study of compounds and reactions. It forms when zinc reacts with sulfuric acid, resulting in a compound that dissolves easily in water. Zinc sulfate is often used in various applications, including as a dietary supplement, in fertilizers to supply essential zinc to plants, and in medicine to treat zinc deficiencies. Understanding zinc sulfate helps to grasp the broader concepts of how different salts and compounds interact in chemical reactions.
| Property | Value |
|---|---|
| Formula | ZnSO₄ |
| Hill Formula | O₄SZn |
| Name | Zinc Sulfate |
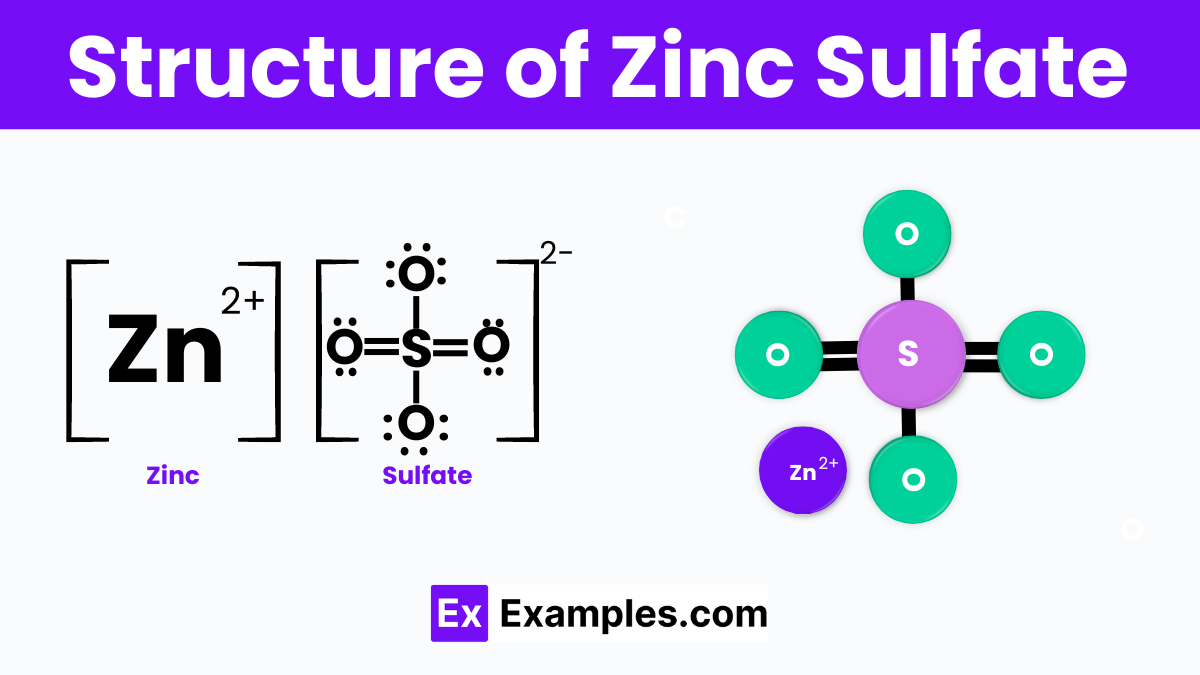
The structure of zinc sulfate consists of one zinc ion (Zn²⁺) and one sulfate ion (SO₄²⁻). In this structure, the zinc ion is bonded to the oxygen atoms of the sulfate ion. The sulfate ion has a tetrahedral shape, with the sulfur atom in the center and four oxygen atoms surrounding it. This arrangement forms a crystalline structure, where each zinc ion coordinates with oxygen atoms from nearby sulfate ions, creating a repeating lattice pattern. This structure allows zinc sulfate to dissolve easily in water, making it useful in various applications.
To prepare zinc sulfate, you can react zinc with sulfuric acid. First, take a piece of zinc metal and add it to a container of dilute sulfuric acid. The zinc reacts with the sulfuric acid to produce zinc sulfate and hydrogen gas. You will notice bubbles forming as the hydrogen gas is released.
The chemical equation for this reaction is:
After the reaction, you can filter the solution to remove any unreacted zinc. Then, evaporate the water from the solution to obtain zinc sulfate crystals. This method is straightforward and is commonly used in laboratories to produce zinc sulfate.
| Property | Description |
|---|---|
| Appearance | Colorless, crystalline solid |
| Solubility | Dissolves easily in water |
| Molecular Formula | ZnSO₄ |
| Molecular Weight | 161.47 g/mol |
| Melting Point | 680°C (decomposes) |
| Density | 3.54 g/cm³ |
| Taste | Astringent, metallic |
| Odor | Odorless |
Zinc sulfate dissolves easily in water, forming an aqueous solution. This property makes it useful in various applications where a liquid form is needed.
ZnSO₄ (s) → ZnSO₄ (aq)
Zinc sulfate reacts with bases like sodium hydroxide (NaOH) to form zinc hydroxide (Zn(OH)₂) and sodium sulfate (Na₂SO₄). This reaction is an example of a double displacement reaction.
ZnSO₄ (aq) + 2NaOH (aq) → Zn(OH)₂ (s) + Na₂SO₄(aq)
Zinc sulfate reacts with carbonates like sodium carbonate (Na₂CO₃) to form zinc carbonate (ZnCO₃) and sodium sulfate. This reaction produces a precipitate of zinc carbonate.
ZnSO₄ (aq) + Na₂CO₃ (aq) → ZnCO₃ (s) + Na₂SO₄ (aq)
| Property | Value |
|---|---|
| CAS Registry Number | 7733-02-0 |
| PubChem Compound ID | 24424 |
| PubChem Substance ID | 24859179 |
| SMILES Identifier | [O-]S(=O)(=O)[O-].[Zn+2] |
| InChI Identifier | InChI=1/H2O4S.Zn/c1-5(2,3)4;/h(H2,1,2,3,4);/q;+2/p-2/fO4S.Zn/q-2;m |
| MDL Number | MFCD00011302 |
Zinc sulfate corrects zinc deficiency in crops. Farmers apply it to soil or leaves, helping plants grow healthier and produce more.
Doctors use zinc sulfate as a dietary supplement to treat zinc deficiency in humans. It helps boost the immune system and improve overall health.
Industries use zinc sulfate in manufacturing processes. It acts as a mordant in dyeing, a preservative for leather, and a component in various chemical products.
Water treatment facilities use zinc sulfate to remove impurities. It helps clarify water by precipitating unwanted particles.
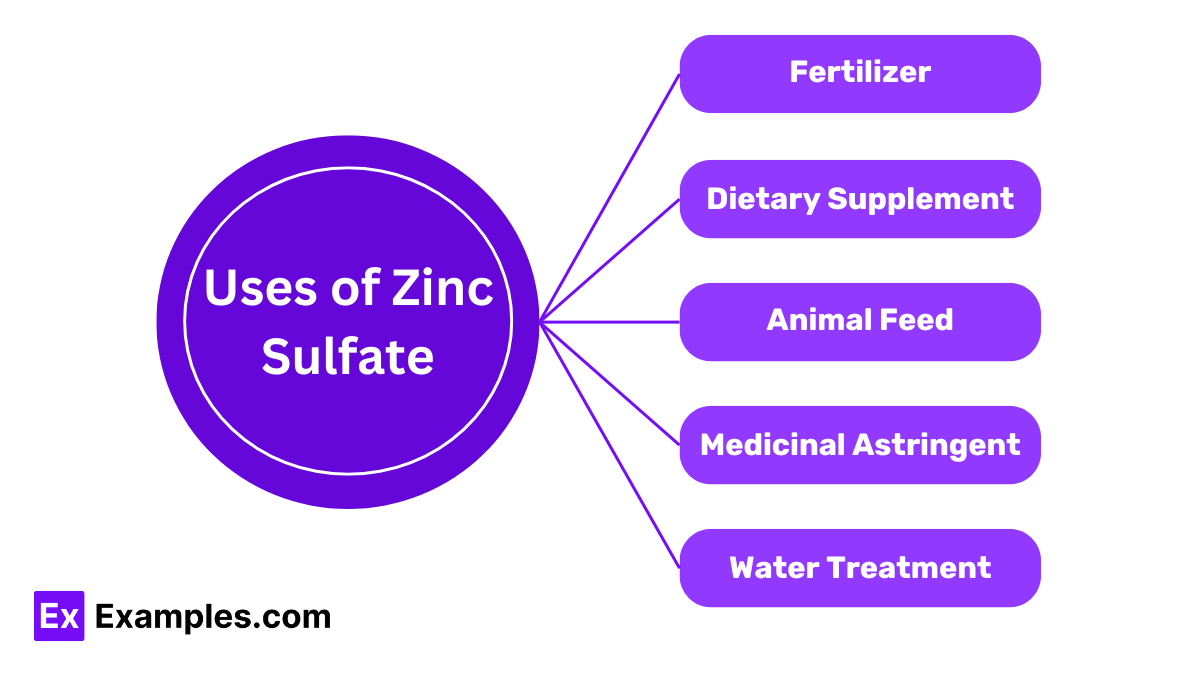
Farmers use zinc sulfate as a fertilizer to correct zinc deficiencies in soil, promoting healthy plant growth and higher crop yields.
Veterinarians add zinc sulfate to animal feed to ensure livestock receive essential nutrients, improving their health and growth.
People take zinc sulfate as a dietary supplement to treat zinc deficiency, boosting the immune system and overall health.
Doctors use zinc sulfate as an astringent in medical treatments. It helps reduce skin irritation and inflammation.
Water treatment plants use zinc sulfate to purify water. It helps remove impurities, making the water safe for consumption.
Textile industries use zinc sulfate as a mordant in dyeing and printing fabrics. It helps fix dyes to the fabric, ensuring vibrant and lasting colors.
Zinc sulfate is good for treating zinc deficiency, promoting plant growth, enhancing animal health, purifying water, and improving skin conditions as an astringent.
Taking zinc sulfate daily is safe in recommended doses, but excessive intake can cause adverse effects. Always follow healthcare advice.
A patient takes zinc sulfate to treat zinc deficiency, boost the immune system, and improve overall health.
Yes, zinc sulfate is highly soluble in water, making it useful for various applications.
People with allergies to zinc, certain medical conditions, or those on specific medications should avoid zinc sulfate. Consult a doctor first.
Disadvantages of zinc sulfate include nausea, vomiting, diarrhea, stomach pain, and potential interactions with medications.
Yes, zinc sulfate has anti-inflammatory properties, which help reduce skin irritation and inflammation.
Avoid taking zinc supplements with high-fiber foods, calcium, iron, or antibiotics as they can interfere with zinc absorption.
Zinc sulfate is an ionic compound, consisting of zinc ions (Zn²⁺) and sulfate ions (SO₄²⁻).
The pH of a zinc sulfate solution is typically acidic, around 4-5.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary use of zinc sulfate in medicine?
To treat hypertension
To supplement zinc deficiency
To reduce cholesterol levels
To treat bacterial infections
Which form of zinc sulfate is most commonly used in dietary supplements?
Zinc sulfate monohydrate
Zinc chloride
Zinc sulfate heptahydrate
Zinc oxide
In which industry is zinc sulfate used to prevent plant diseases?
Textile
Agriculture
Pharmaceuticals
Construction
What is the chemical formula of zinc sulfate?
ZnSO4
ZnCl2
ZnO
ZnCO3
Zinc sulfate can be used in which of the following treatments?
Acne
Heart disease
Asthma
Kidney stones
How is zinc sulfate typically administered to treat deficiencies?
Intravenously
Topically
Orally
By inhalation
Which property of zinc sulfate makes it effective as an astringent in skin care?
Its solubility
Its acidic nature
Its basic nature
Its high pH
What is a common side effect of taking zinc sulfate supplements?
Constipation
Dry skin
Nausea
Headache
Zinc sulfate is used in which type of solution for treating eye infections?
Saline solution
Antiseptic solution
Hypertonic solution
Zinc sulfate solution
What is one of the main industrial uses of zinc sulfate?
Production of batteries
Dyeing fabrics
Manufacturing glass
As a pesticide
Before you leave, take our quick quiz to enhance your learning!

