What is the atomic number of Boron?
3
5
7
9
Boron, a fascinating element, plays a pivotal role in diverse fields, from agriculture to industry. This comprehensive guide is tailored for educators, aiming to demystify Boron’s complexities. Here, we’ll explore its multifaceted nature, offering practical insights and engaging examples. Ideal for teachers, this resource simplifies Boron’s concepts, making them accessible and intriguing for both you and your students. Embrace this journey into the world of Boron and elevate your teaching approach!
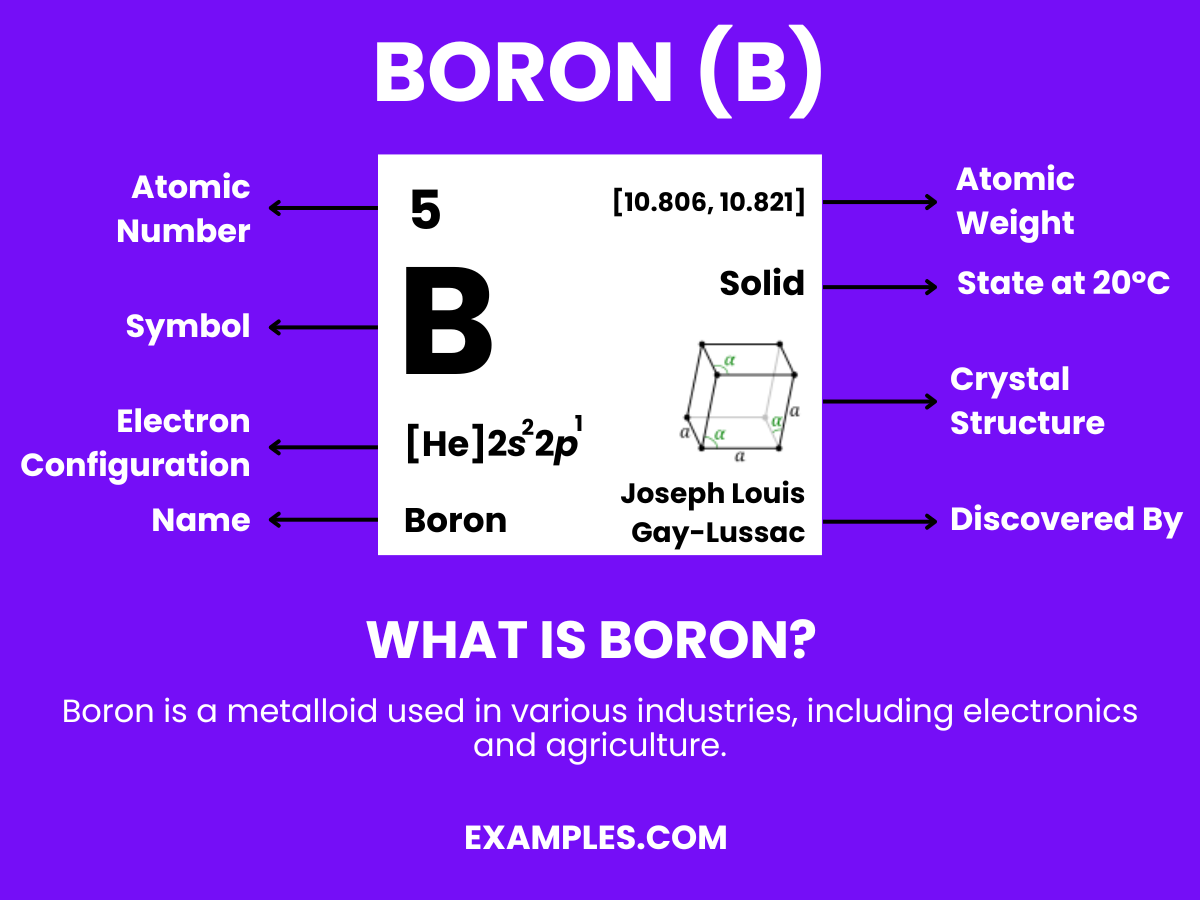
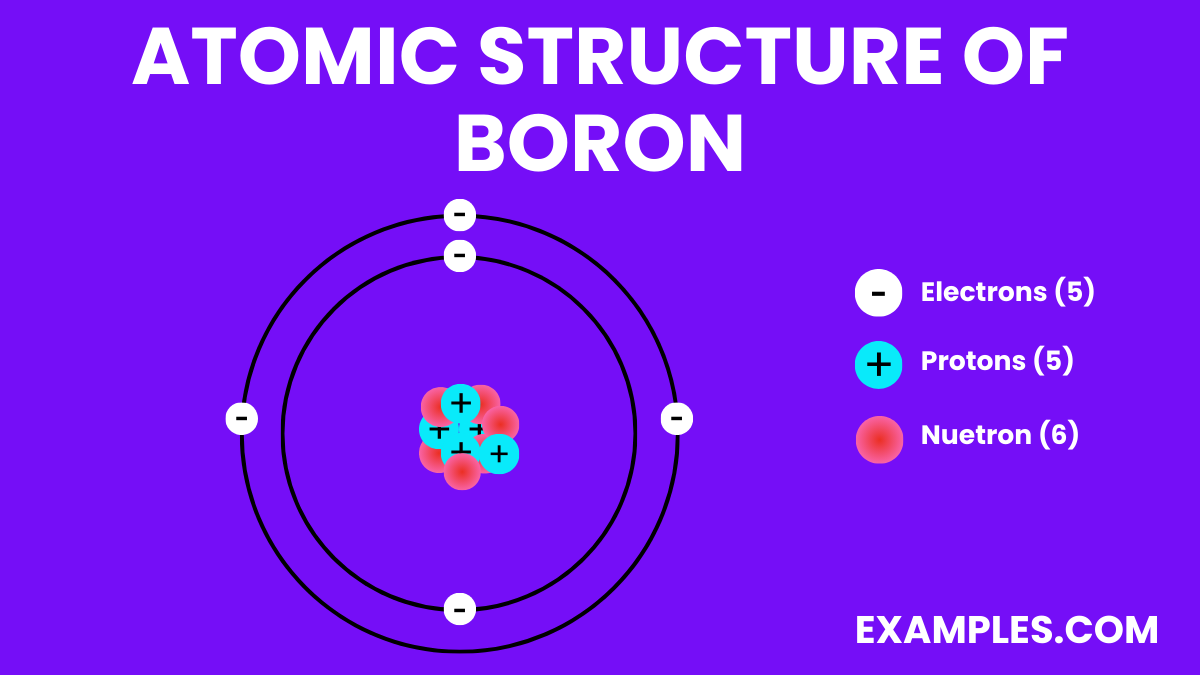
Boron is a unique chemical element, represented by the symbol ‘B’ and atomic number 5. Found in the Earth’s crust and solar system, it’s crucial for plant growth and has significant applications in glass and ceramics production. Unlike metals, Boron is a metalloid, exhibiting properties of both metals and non-metals. This duality makes it an intriguing subject in science education, offering a rich context for teaching about chemical elements and their diverse roles. Understanding Boron helps students grasp fundamental scientific concepts and encourages curiosity about the natural world.
| Silicon (Si) |
| Germanium (Ge) |
| Arsenic (As) |
| Antimony (Sb) |
| Tellurium (Te) |
| Property | Description |
|---|---|
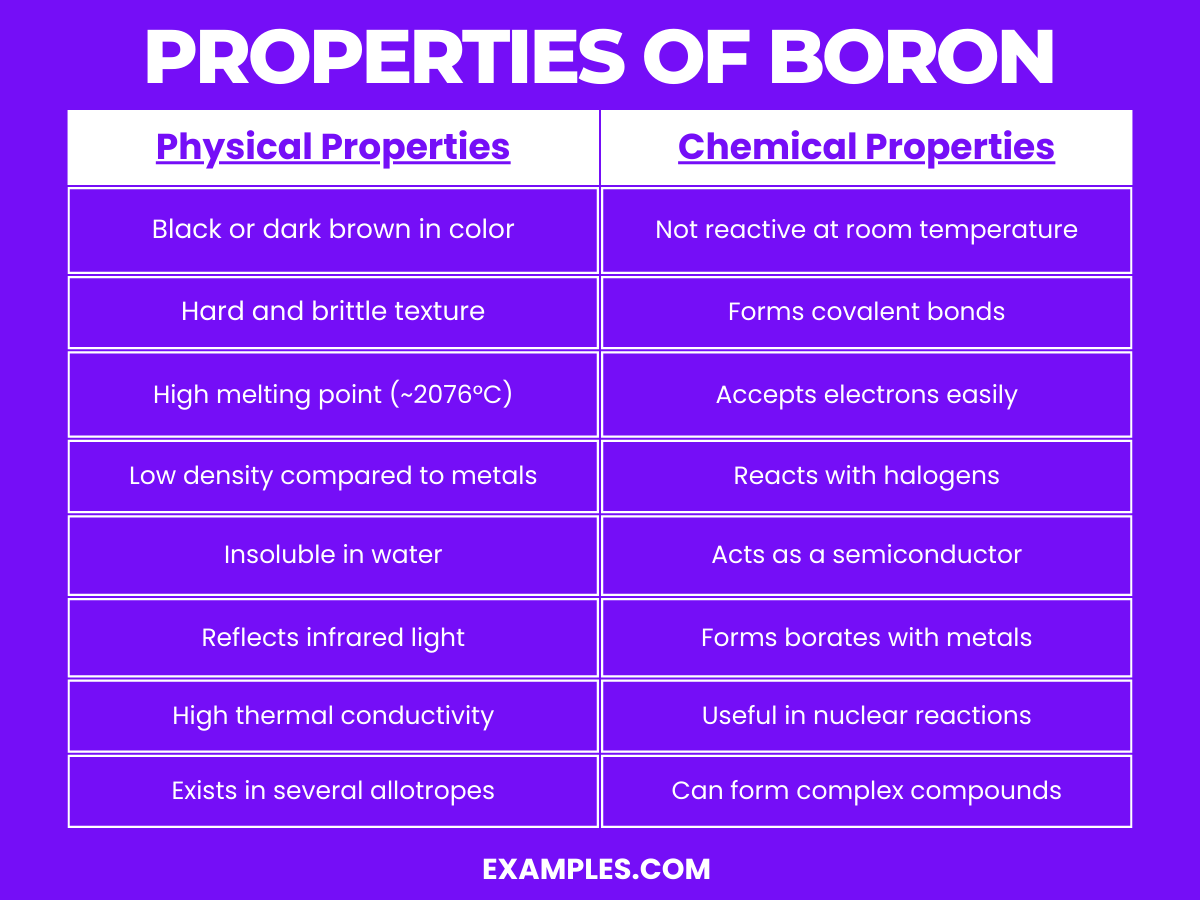
| Appearance | Black or dark brown, depending on the allotrope |
| Texture | Hard and brittle |
| Melting Point | High, at approximately 2076°C (3769°F) |
| Density | Lower than most metals; about 2.34 g/cm³ |
| Solubility in Water | Insoluble |
| Thermal Conductivity | High, efficiently conducts heat |
| Allotropes | Exists in several forms, including amorphous and crystalline |
| Optical Properties | Reflects infrared light |
Boron, a unique element in the periodic table, displays several interesting chemical properties:
| Property | Value |
|---|---|
| Atomic Number | 5 |
| Atomic Weight | 10.81 g/mol |
| Phase at Room Temperature | Solid |
| Melting Point | 2076°C (3769°F) |
| Boiling Point | 3927°C (7101°F) |
| Specific Heat Capacity | 1.026 J/(g·K) at 25°C |
| Thermal Conductivity | 27 W/(m·K) |
| Property | Value |
|---|---|
| Crystal Structure | Rhombohedral (α-Boron), Tetragonal (β-Boron) |
| Density | ~2.34 g/cm³ (α-Boron), ~2.46 g/cm³ (β-Boron) |
| Mohs Hardness | ~9.3 |
| Young’s Modulus | ~400 GPa |
| Electrical Resistivity | ~1×10⁶ μΩ·m (at room temperature) |
| Thermal Expansion Coefficient | 5-7 µm/(m·K) |
| Property | Value |
|---|---|
| Electronegativity (Pauling scale) | ~2.04 |
| Electron Affinity | ~26.7 kJ/mol |
| Electrical Conductivity | Insulator (α-Boron), Semiconductor (β-Boron) |
| Magnetic Susceptibility | Diamagnetic |
| Property | Value |
|---|---|
| Isotopes | Boron-10 (¹⁰B), Boron-11 (¹¹B) |
| Natural Abundance | ¹⁰B: ~19.9%, ¹¹B: ~80.1% |
| Cross Section for Thermal Neutrons | ¹⁰B: High (3840 barns for absorption), ¹¹B: Low (0.0055 barns) |
| Nuclear Spin | ¹⁰B: 3, ¹¹B: 3/2 |
Boron, a versatile element in the periodic table, forms various compounds with significant industrial and scientific applications. Here, we explore the top six chemical compounds of boron, complete with their relevant equations.
Formula: H₃BO₃
Preparation: B₂O₃ + 3H₂O → 2H₃BO₃
Uses: Antiseptic, insecticide, flame retardant, neutron absorber in nuclear reactors.
Formula: Na₂B₄O7·10H₂O
Preparation: Na2CO₃ + 4B(OH)₃ + 5H₂O → Na₂B₄O7·10H₂O + CO₂
Uses: Detergent, water softening agent, buffer in biochemistry.
Formula: BN
Preparation: B₂H₆ + 6NH₃ → 2BN + 12H₂
Uses: High-temperature lubricant, electrical insulators, structural material for aerospace applications.
Formula: B₄C
Preparation: B₂O₃ + 3C → B₄C + CO₂
Uses: Abrasives, armor plating, neutron absorber in nuclear reactors.
Formula: BF₃
Preparation: B₂O₃ + 6HF → 2BF₃ + 3H₂O
Uses: Catalyst in organic synthesis, flame retardant, high-energy fuel additive.
Formula: B₂O₃
Preparation: 2B + 3O₂ → B₂O₃
Uses: Glass and fiberglass production, flux in metallurgy, fire retardant.
Boron has two naturally occurring isotopes, each with distinct properties. The table below provides a detailed overview:
| Isotope | Symbol | Atomic Mass (u) | Natural Abundance (%) | Nuclear Spin |
|---|---|---|---|---|
| Boron-10 | ¹⁰B | 10.0129 | 19.9 | 3 |
| Boron-11 | ¹¹B | 11.0093 | 80.1 | 3/2 |
Boron-10 is notable for its high neutron cross-section, making it useful in nuclear reactors and radiation shields. Boron-11, the more abundant isotope, is used in nuclear magnetic resonance (NMR) spectroscopy due to its favorable nuclear spin properties.
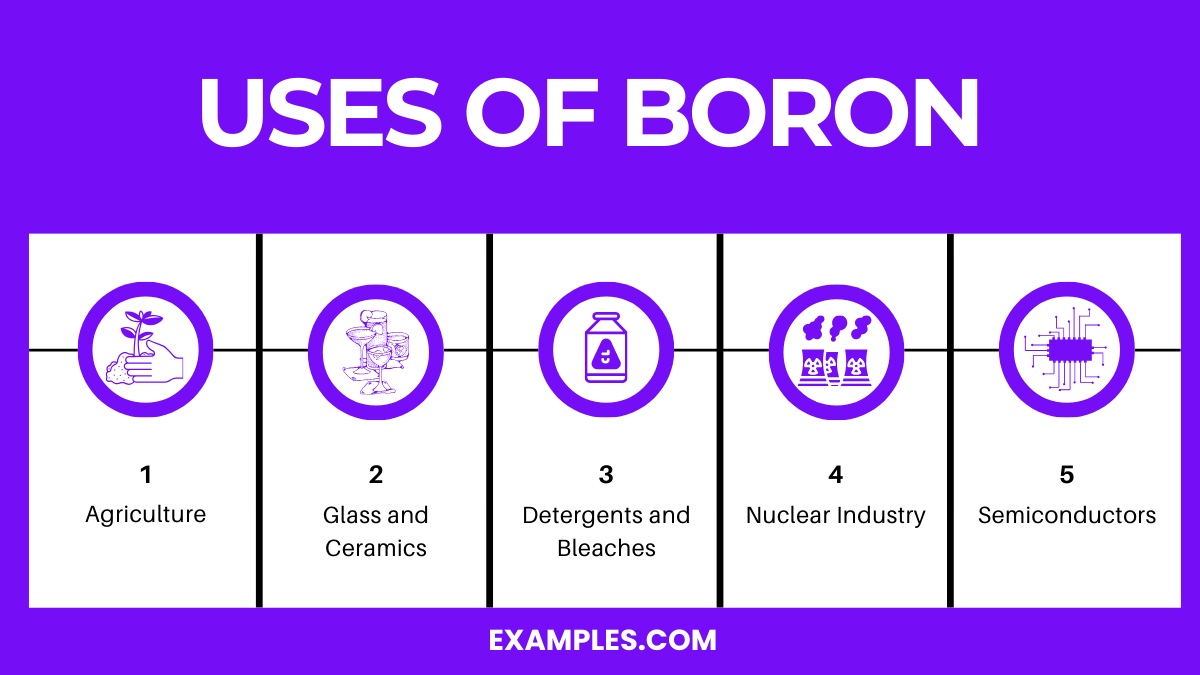
Boron, a multifaceted element, plays a crucial role in various industries due to its unique chemical and physical properties. Here are the top five uses of boron:
Boron is vital for plant growth and is often added to fertilizers. It aids in the development and strengthening of plant cell walls, enhancing nutrient transport. Boron deficiency in soil can lead to stunted plant growth, making its addition essential in agriculture.
In the glass industry, boron is used to create borosilicate glass, which is more resistant to thermal shock than ordinary glass. This makes it ideal for use in heat-resistant glassware and fiberglass. In ceramics, boron helps reduce the melting temperature and improves the durability and thermal resistance of the products.
Boron compounds, especially borax, are used in laundry and cleaning products. They enhance the cleaning power of detergents by softening water and helping to break down organic stains and grime.
Boron, particularly Boron-10 isotope, is an effective neutron absorber. It is used in control rods of nuclear reactors to control the nuclear fission process. Its presence helps in regulating the energy output and ensuring the safe operation of the reactor.
Boron is used as a doping agent in the manufacturing of semiconductors. It imparts p-type conductivity to silicon and other semiconductor materials. This is crucial in creating the diodes and transistors found in electronic devices.
The commercial production of boron involves several steps, primarily focusing on the extraction and refining of boron-containing minerals.
Extraction of Borate Minerals: The most common boron-bearing minerals are borax, kernite, and ulexite. These are usually extracted through surface or underground mining. Surface mining is more common, where large deposits are stripped away, and the minerals are extracted.
Refining Process: Once extracted, the crude boron minerals undergo a refining process to increase the boron content. The refining process varies based on the mineral. For instance, borax is refined through recrystallization, while kernite is refined by dissolution in hot water, followed by recrystallization.
Conversion to Boric Acid or Boron Oxide: The refined boron compounds are often converted to more commercially useful forms like boric acid (H3BO3) or boron oxide (B2O3). This involves chemical processes like acidification or heating at high temperatures.
Production of Boron Alloys and Compounds: Finally, pure boron or its compounds are used to produce various alloys and compounds. For instance, boron is added to steel and other metals to improve their hardness and corrosion resistance. Boron compounds are also synthesized for use in a wide range of industrial applications.
The production of boron is a complex process, requiring careful handling and processing to ensure the purity and quality of the final products. The majority of the world’s boron is produced in the United States and Turkey, with these countries having the largest boron mineral deposits.
Boron, a naturally occurring element found in the environment, food, and water, has various health effects, both beneficial and potentially harmful, depending on the exposure level.
The health effects of boron largely depend on the dose and exposure duration. While trace amounts are essential for health, excessive exposure can lead to adverse health effects.
Boron is both beneficial and potentially harmful to the environment, depending on its concentration and the form in which it is present.
Boron boosts bone health, aids hormonal balance, and enhances cognitive function. It’s crucial for nutrient absorption and overall well-being.
Boron is used in agriculture, glass and ceramics production, detergents, nuclear industry, and semiconductors. It’s versatile in industrial and technological applications.
Boron is found in apples, oranges, nuts, potatoes, avocados, and leafy greens. A varied diet usually provides sufficient boron intake.
For men, boron supports testosterone production, enhances bone strength, and plays a role in mental clarity and cognitive function.
Boron is commonly found in the Earth’s crust, boron-rich minerals like borax and kernite, everyday foods, detergents, fiberglass, and ceramics.
Boron’s diverse applications, from agriculture to industrial uses, highlight its importance. Its benefits in plant growth and in making glass and ceramics are notable. Awareness of boron’s role and safe handling practices are key in harnessing its benefits while minimizing risks.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Boron?
3
5
7
9
Which property is Boron well-known for?
High ductility
High electrical conductivity
High thermal stability
High reactivity with nitrogen
Boron is primarily classified as a:
Metal
Non-metal
Metalloid
Halogen
What is the primary use of Boron in the glass industry?
Coloring agent
Strengthening agent
Thinning agent
Clarifying agent
Which form of Boron is considered highly pure and is used in semiconductor industry?
Amorphous boron
Boric acid
Boron carbide
Boron nitride
What type of bond is primarily formed by Boron compounds?
Ionic
Covalent
Metallic
Hydrogen
Boron is an essential nutrient for which type of organisms?
Mammals
Plants
Bacteria
Fungi
The discovery of Boron was made by:
Humphry Davy
Joseph Priestley
Antoine Lavoisier
Sir Humphry Davy and Joseph-Louis Gay-Lussac
Boron trifluoride is used as a:
Refrigerant
Catalyst
Plasticizer
Flame retardant
In which form is Boron typically found in nature?
Elemental boron
Boron nitride
Boric acid
Borax
Before you leave, take our quick quiz to enhance your learning!

