What is steel primarily made of?
Iron and copper
Iron and carbon
Aluminum and carbon
Iron and magnesium
Steel is a remarkable metallic compound that stands at the heart of modern construction and manufacturing, embodying the strength and versatility demanded by today’s world. Born from the union of iron and carbon, this chemistry marvel showcases a variety of properties by incorporating different amounts of carbon and other elements, leading to its widespread use in everything from towering skyscrapers to everyday household tools. The process of making steel, blending these elements at high temperatures, not only showcases human ingenuity in manipulating chemical compositions for our benefit but also highlights steel’s pivotal role in shaping the infrastructure and technologies that define our contemporary lifestyle.
Carbon steel is a type of steel where the main alloying element is carbon. It comes in three subcategories: low, medium, and high carbon steel. Low carbon steel, also known as mild steel, contains a small percentage of carbon, making it more ductile and malleable. It’s commonly used in construction and for manufacturing car bodies due to its strength and flexibility. Medium carbon steel has a higher amount of carbon, offering a balance between ductility, strength, and wear resistance, making it ideal for gears and structural components. High carbon steel, with even more carbon, is very hard and strong, used for cutting tools and blades.
Alloy steel is made by adding various elements to the mix, such as chromium, nickel, and molybdenum, to improve its mechanical properties. These additions can make the steel more durable, easier to work with, or resistant to corrosion and heat. Alloy steels are divided into two groups: low alloy steels, which have a small percentage of alloying elements, and high alloy steels, which have a high percentage. They’re used in a wide range of applications, from pipelines and automotive parts to power generation equipment and military hardware.
Stainless steel is best known for its corrosion resistance, thanks to a significant chromium content (at least 10.5%). This type of steel can be divided into several groups based on its crystalline structure, such as austenitic, ferritic, and martensitic, each with unique properties and uses. Austenitic stainless steel is the most common, known for its weldability and formability, making it perfect for kitchen utensils, appliances, and medical equipment. Ferritic stainless steel is magnetic and has good resistance to stress corrosion cracking, used in automotive parts and appliances. Martensitic stainless steel is hardenable by heat treatments, making it suitable for cutlery and tools.
Tool steel contains various alloying elements like tungsten, molybdenum, cobalt, and vanadium, which increase its heat resistance and durability. This type of steel is designed to be made into tools used for cutting, pressing, and molding materials. Its ability to withstand high temperatures without losing hardness makes it ideal for manufacturing drills, cutters, molds, and dies. Tool steel is categorized into several types, including water-hardening, air-hardening, and oil-hardening, each offering different characteristics suited to specific applications.
Steel’s structure is a mixture of iron atoms with a small amount of carbon atoms, usually up to 2%, which greatly enhances its strength. This combination forms a crystal lattice that gives steel its unique properties, such as toughness, durability, and the ability to be shaped or welded. When steel is made, the iron and carbon atoms arrange themselves into a specific pattern within the metal, depending on how the steel is cooled and treated. This internal structure can be altered by adding other elements or by heat treatment, leading to various types of steel with different characteristics suitable for a wide range of applications. The precise arrangement and bonding of atoms within steel make it an indispensable material in construction, manufacturing, and countless other industries.
Steel is prepared primarily through two processes: the blast furnace method and the electric arc furnace method.
In this method, iron ore (iron oxide) is reduced to iron by heating it with coke (a form of carbon) and limestone in a large furnace. The chemical equation for this reduction process can be simplified as:
Here, the iron oxide reacts with carbon, producing liquid iron and carbon monoxide gas. This liquid iron is then mixed with a specific amount of carbon (and sometimes other elements) to make different types of steel.
In this method, recycled steel scrap is melted using high-power electric arcs between carbon electrodes in a furnace. This process does not directly involve chemical equations like the blast furnace method since it’s more about melting the scrap steel and then adding the required amount of carbon and other elements to achieve the desired steel composition. After melting and mixing, the steel is cast into various shapes and can undergo further processes, such as rolling, to form it into products like beams, sheets, or bars. This method is more flexible and environmentally friendly, as it recycles existing steel and consumes less energy.
| Property | Description |
|---|---|
| Strength | Steel is very strong, making it capable of withstanding significant force or weight without breaking. |
| Durability | It’s highly durable and can last for many years without deteriorating, even in harsh conditions. |
| Malleability | Steel can be shaped and bent into various forms without breaking, allowing for versatile use in construction and manufacturing. |
| Ductility | This property means steel can be stretched into wires or thin rods without snapping, useful in applications requiring flexibility. |
| Conductivity | Steel conducts electricity, though not as well as metals like copper, making it suitable for certain electrical applications. |
| Density | Steel is dense, giving it a high strength-to-volume ratio that supports heavy loads and structures. |
| Melting Point | It has a high melting point, generally around 1370°C to 1510°C (2500°F to 2750°F), which means it can withstand high temperatures without losing its form. |
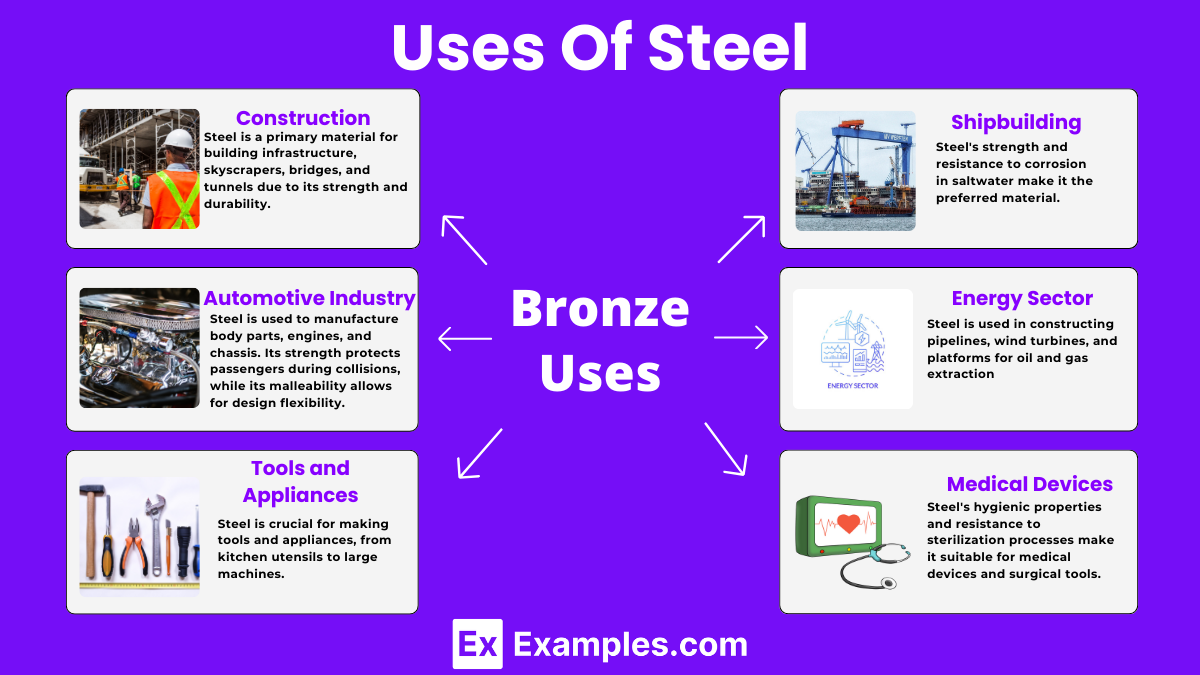
Steel is a primary material for building infrastructure, skyscrapers, bridges, and tunnels due to its strength and durability. It supports structures under heavy loads and withstands environmental stresses.
In the automotive industry, steel is used to manufacture body parts, engines, and chassis. Its strength protects passengers during collisions, while its malleability allows for design flexibility.
Steel is crucial for making tools and appliances, from kitchen utensils to large machines. Its ability to retain an edge and resist wear makes it ideal for knives, while its thermal properties are perfect for appliances.
For shipbuilding, steel’s strength and resistance to corrosion in saltwater make it the preferred material. It is used in the hulls, decks, and superstructures of marine vessels.
In the energy sector, steel is used in constructing pipelines, wind turbines, and platforms for oil and gas extraction. Its durability ensures long-term performance in harsh environments.
Steel’s hygienic properties and resistance to sterilization processes make it suitable for medical devices and surgical tools, ensuring safety and reliability in medical treatments.
In the food industry, stainless steel is used for cooking tools, machinery, and storage containers due to its resistance to corrosion and ease of cleaning, preventing food contamination.
Steel is known for its exceptional strength, making it ideal for supporting structures in buildings, bridges, and vehicles. Its robustness ensures safety and longevity.
Steel’s durability means it can withstand wear, pressure, or damage, making it suitable for long-term applications in construction and manufacturing.
Thanks to various alloying techniques, steel can be adapted for a wide range of uses, from heavy industrial machinery to delicate precision instruments.
Steel is one of the most recycled materials in the world. Its properties remain unchanged no matter how many times it is recycled, making it a sustainable choice.
Compared to other building materials, steel offers economic efficiency due to its durability and the speed of construction it enables, reducing overall project costs.
Steel is resistant to many natural and industrial elements, including moisture, fire, and chemicals, ensuring safety and integrity of structures.
Modern steel making processes are highly energy-efficient, with significant reductions in energy use and carbon dioxide emissions over the past decades, contributing to environmental conservation.
Steel is called a metal because it is a strong, hard substance made from iron and carbon, possessing metallic properties like conductivity and malleability.
Yes, steel is primarily iron, enhanced with a small amount of carbon (up to 2%) and sometimes other elements for improved strength and durability.
Tungsten is considered the strongest metal on earth, known for its high tensile strength and melting point, surpassing even steel and iron.
Steel is stronger than iron. The addition of carbon and other elements to iron significantly improves its strength, durability, and resistance to corrosion.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is steel primarily made of?
Iron and copper
Iron and carbon
Aluminum and carbon
Iron and magnesium
What is the typical percentage of carbon in most steels?
0.1-0.5%
1-2%
5-10%
10-20%
Which process is commonly used to remove impurities from iron to make steel?
Smelting
Refining
Bessemer process
Casting
Which of the following is a characteristic property of stainless steel?
High rust resistance
High electrical conductivity
High magnetism
Low tensile strength
Which element is primarily responsible for the corrosion resistance of stainless steel?
Carbon
Chromium
Iron
Copper
What is the effect of adding carbon to steel?
It makes the steel more flexible
It makes the steel harder and stronger
It decreases the strength of steel
It makes the steel brittle
Which type of steel is typically used in construction and infrastructure?
Stainless steel
Carbon steel
Tool steel
Alloy steel
What happens when the carbon content in steel exceeds 2%?
It becomes stainless steel
It becomes cast iron
It becomes a soft metal
It becomes more ductile
Which of the following is a major disadvantage of steel?
Low durability
Susceptibility to corrosion
Low melting point
Low availability
What is the purpose of annealing steel?
To make it harder
To make it more flexible
To improve its electrical properties
To remove impurities
Before you leave, take our quick quiz to enhance your learning!

