What is the primary use of tin in modern industries?
Wiring
Solder
Building construction
Pharmaceuticals
Tin, a key element in the periodic table, holds a special place in both historical and modern contexts. This guide delves into the fascinating world of Tin, offering educators valuable insights and examples to enrich their teaching. Known for its malleability and resistance to corrosion, Tin is more than just a material; it’s a gateway to understanding fundamental concepts in chemistry and physics. By incorporating real-life applications and historical perspectives, this article aims to provide teachers with engaging content that sparks curiosity and learning about Tin.
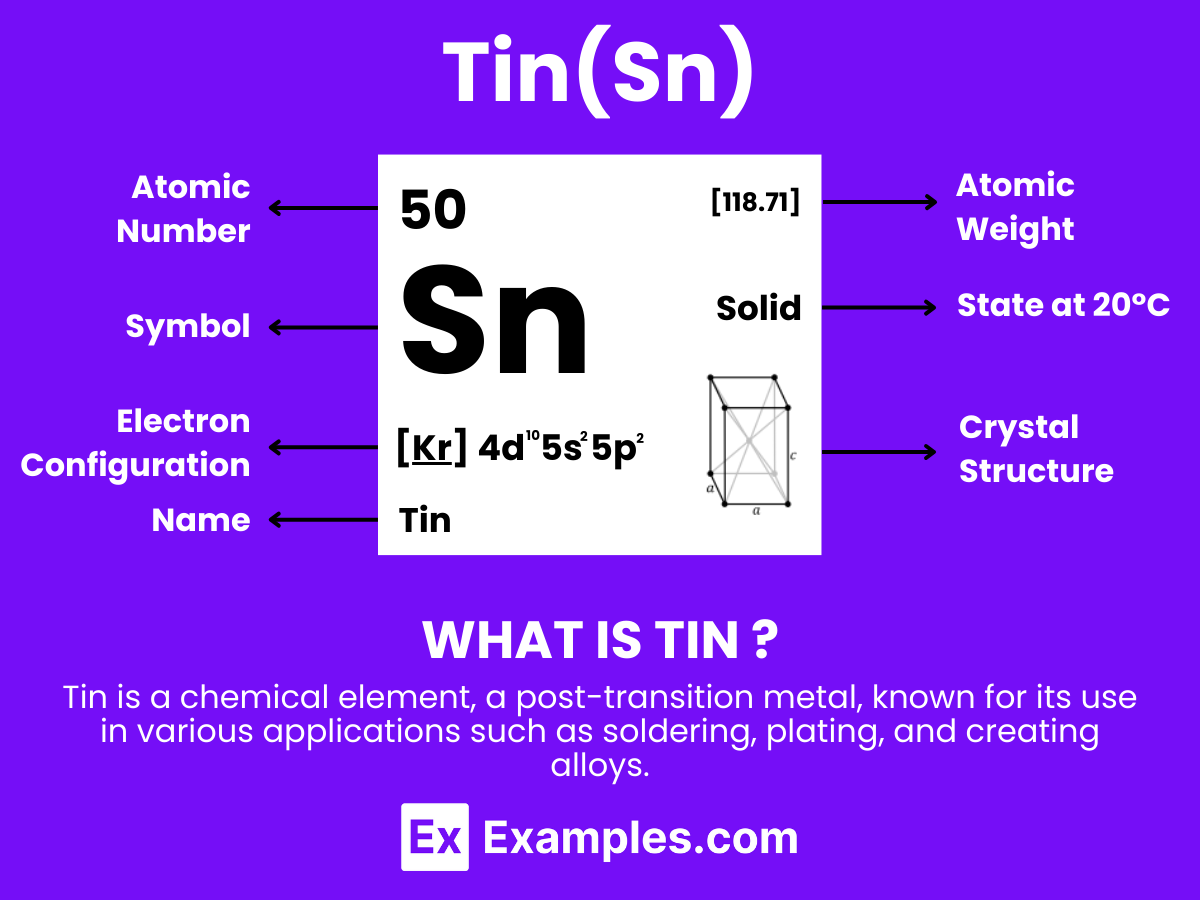
Tin is a chemical element with the symbol Sn (from Latin: stannum) and atomic number 50. It is a post-transition metal known for its silvery-white appearance and high malleability. Tin is not easily oxidized in air, making it resistant to corrosion and ideal for coating other metals. Historically, Tin has been used in alloys such as bronze, and today, it finds applications in solder, tin plating, and various other industries. For teachers, Tin serves as an excellent example to discuss the properties of elements, their place in the periodic table, and their practical applications in everyday life.
Tin (Sn), a chemical element in the carbon group with atomic number 50, exhibits fascinating atomic characteristics that have been pivotal in various technological and industrial applications throughout history. Here’s an overview of the atomic structure of tin:
Neutrons: The number of neutrons in tin can vary, leading to different isotopes of the element. Tin is unique in having a significant number of stable isotopes, the most of any element, with ten stable isotopes ranging from tin-112 to tin-124. These isotopes have varying numbers of neutrons, from 62 to 74, affecting the atomic mass but not the chemical properties of the element.
Electron Shells: Tin’s electrons are distributed across five shells, following the 2, 8, 18, 18, 4 pattern. The valence electrons (those in the outermost shell) play a crucial role in chemical reactions and the formation of compounds.
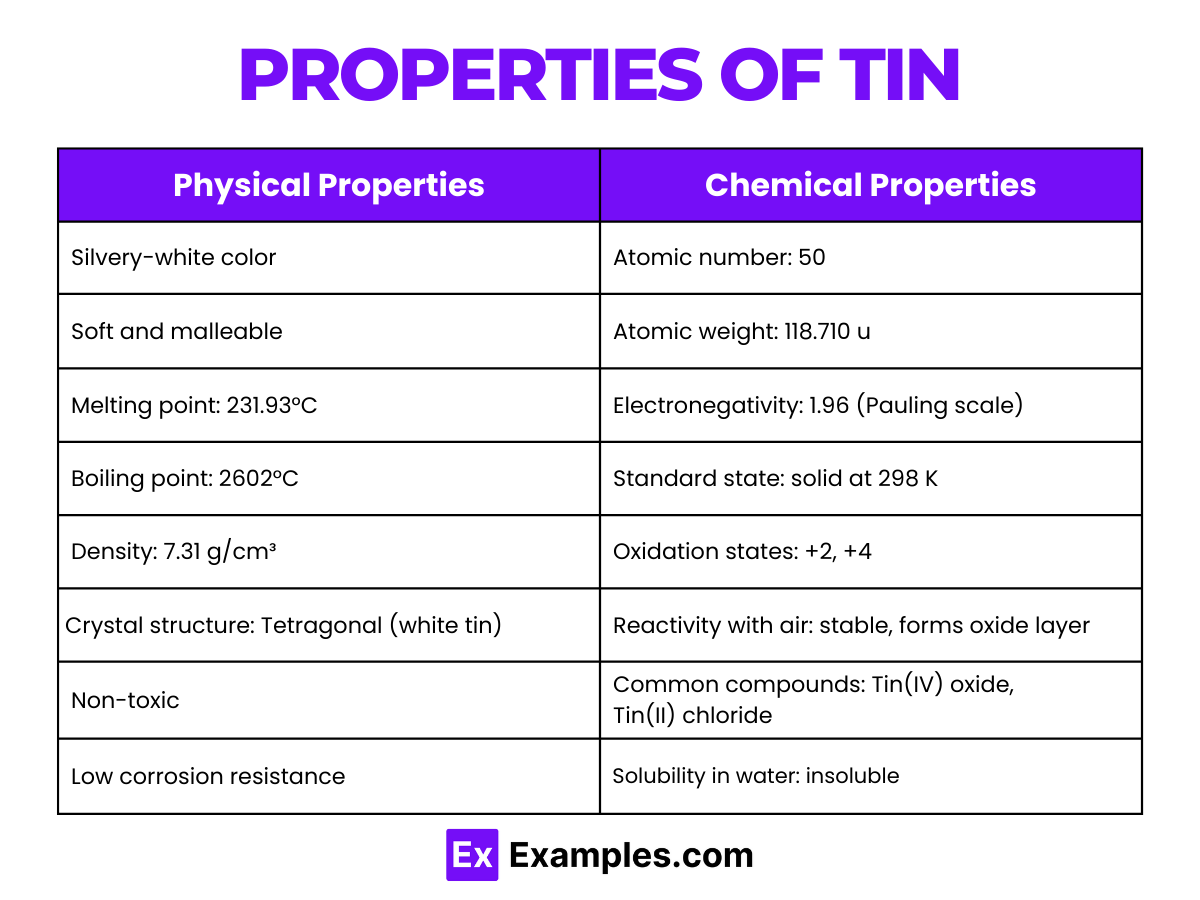
Chemical and Physical Properties: The electron configuration and the distribution of electrons across its shells endow tin with characteristic properties, such as malleability, ductility, and a relatively low melting point for a metal. Tin can exist in two allotropic forms at normal pressure: white tin, which is metallic, and gray tin, which is nonmetallic at temperatures below 13.2°C (55.8°F)
| Property | Description |
|---|---|
| Appearance | Silvery-white, lustrous metal |
| Atomic Mass | Approximately 118.71 u |
| Density | 7.31 g/cm³ at room temperature |
| Melting Point | 231.93°C (449.47°F) |
| Boiling Point | 2602°C (4716°F) |
| Electrical Conductivity | Good conductor of electricity |
| Thermal Conductivity | High thermal conductivity |
| Malleability and Ductility | Highly malleable and ductile |
| Crystal Structure | Tetragonal (white tin) at room temperature; cubic (gray tin) below 13.2°C |
These physical properties underline tin’s utility in various applications, including electronics, coatings, and alloy production.
Tin exhibits a range of chemical behaviors due to its electron configuration and position in the periodic table. Here’s an in-depth look at its chemical properties:
| Property | Value |
|---|---|
| Melting Point | 231.93°C (449.47°F) |
| Boiling Point | 2602°C (4716°F) |
| Heat of Fusion | 7.03 kJ/mol |
| Heat of Vaporization | 296.1 kJ/mol |
| Specific Heat Capacity | 27.112 J/(mol·K) |
| Thermal Conductivity | 66.8 W/(m·K) |
| Property | Value |
|---|---|
| Density | 7.31 g/cm³ |
| Young’s Modulus | 50 GPa |
| Tensile Strength | 14-200 MPa |
| Hardness | 1.5 Mohs |
| Malleability | High |
| Ductility | High |
| Property | Value |
|---|---|
| Electrical Conductivity | 8.69 × 10⁶ S/m |
| Magnetic Susceptibility | -3.8 × 10⁻⁵ |
| Property | Description |
|---|---|
| Atomic Number | 50 – Tin has 50 protons in its nucleus, defining its chemical properties. |
| Atomic Mass | 118.71 u – The average mass of the tin atom, reflecting the sum of its protons and neutrons. |
| Isotopes | Stable: 10 – Tin has the largest number of stable isotopes among all elements, contributing to its diverse applications. |
| Neutron Cross Section | 0.626 barns – A measure of the probability of neutron capture, relevant in nuclear reactions and applications. |
| Half-life of Most Stable Isotope | ^{126}Sn, 2.3 × 10⁵ years – Longest-lived radioactive tin isotope, though most natural tin is stable. |
Tin, symbolized as Sn (from the Latin ‘stannum’), is commonly extracted from its primary ore, cassiterite (SnO₂), through a smelting process that involves several key steps to produce pure tin metal. The preparation of tin can be summarized as follows:
This multi-step process ensures that the tin produced is of high purity, suitable for various industrial and commercial applications.
Tin, a versatile metal, forms numerous compounds exhibiting diverse chemical properties. Here are six significant chemical compounds of tin, along with their descriptions and relevant chemical equations:
Tin is unique among all elements for having the largest number of stable isotopes, with ten recognized by the scientific community. These isotopes range from tin-112 to tin-124, differing in their number of neutrons. Here’s a brief overview of tin’s isotopes:
The diversity of tin’s isotopes is valuable for research in nuclear physics and isotopic geochemistry, providing insights into processes ranging from the formation of solar systems to the behavior of materials under extreme conditions
Tin’s physical and chemical properties make it an essential material in various applications across multiple industries. Here are some of the primary uses of tin:
The commercial production of tin involves several steps, from mining tin ore to refining and processing it into usable forms. The primary source of tin is cassiterite (tin oxide, SnO2), from which tin is extracted through the following processes:
Tin, in its metallic form, is relatively non-toxic and poses little risk to human health. However, certain tin compounds and organotin compounds can have harmful health effects:
Proper handling and industrial hygiene practices are essential to minimize exposure and prevent health risks associated with tin compounds.
Tin’s environmental impact is primarily associated with mining and processing operations. These effects can include:
Tin is called “Sn” because the symbol derives from the Latin name for tin, “stannum.” This nomenclature is part of the international system of chemical symbols used to represent elements based on their Latin names, facilitating universal understanding and communication in the scientific community
Tin is used in a wide array of applications, including soldering materials for electronics, coatings for steel cans to prevent corrosion, glass-making, and creating various alloys like bronze. Its versatility stems from its corrosion resistance, malleability, and low melting point, making it invaluable in industrial and consumer products
Tin is a pure metal in its elemental form, classified under the category of metals in the periodic table. It exists naturally in the Earth’s crust, primarily obtained from the mineral cassiterite (SnO2), and can be processed into a highly pure state for various commercial and industrial applications
In the periodic table, tin is represented by the symbol “Sn” and has an atomic number of 50. It is situated in group 14, also known as the carbon group. This positioning reflects its chemical properties, including the ability to form compounds in multiple oxidation states and its role as a post-transition metal
Tin is a versatile metal with widespread applications in electronics, coatings, and alloys, owing to its distinct physical and chemical properties. While its commercial production is crucial for various industries, attention to the health and environmental impacts of tin and its compounds is essential. Responsible management and recycling can mitigate these effects, sustaining its valuable role in technological advancements and daily use.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary use of tin in modern industries?
Wiring
Solder
Building construction
Pharmaceuticals
Which alloy of tin is commonly used for coating to prevent corrosion?
Bronze
Brass
Pewter
Tinplate
What environmental issue is associated with tin mining?
Deforestation
Air pollution
Water pollution
Soil erosion
In which historical period was tin first used?
Iron Age
Bronze Age
Neolithic
Medieval
What property of tin makes it useful in tin cry?
Elasticity
Brittleness at low temperatures
High melting point
Malleability
What is a common application of tin in the food industry?
Flavor enhancer
Coloring agent
Preservative
Can coating
How is tin most commonly extracted?
Leaching
Smelting
Electrolysis
Distillation
Which of the following is a characteristic feature of organotin compounds?
Solubility in water
Stability in high temperatures
Biodegradability
Use as biocides
What impact does tin have when released into freshwater ecosystems?
Increases algae growth
Harms aquatic plants
Disrupts fish reproduction
None of the above
What global organization regulates the trade and use of tin?
United Nations
World Trade Organization
International Tin Association
Global Mining Consortium
Before you leave, take our quick quiz to enhance your learning!

