What is the primary chemical formula for rust, a common form of iron oxide?
FeO
Fe₂O₃
Fe₃O₄
FeO₂


Iron(III) oxide is also known as ferric oxide. It is a chemical compound made of iron and oxygen with the formula Fe₂O₃. In chemistry, we often see it as a reddish-brown powder commonly found in rust and used as a pigment. It forms when iron reacts with oxygen in the air, creating a coating that protects the iron underneath from further corrosion. Iron(III) oxide is significant in both chemistry and industry, playing a crucial role in the production of iron and steel, and it’s also used in magnetic materials and polishing compounds.
| Property | Value |
|---|---|
| Formula | Fe₂O₃ |
| Name | Iron(III) Oxide |
| Alternate Names | Diiron Trioxide, Ferric Oxide, Haematite, Hematite, Iron Sesquioxide, Oxo-(Oxoferriooxy)iron, Red Iron Oxide, Specularite |
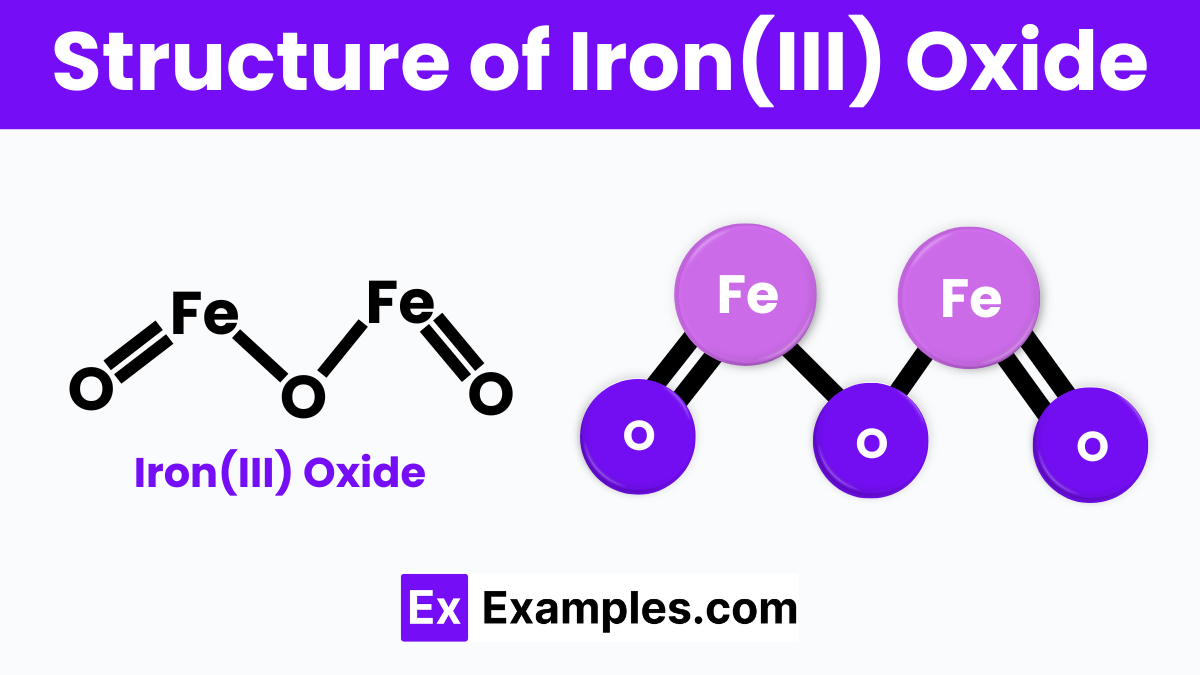
The structure of Iron(III) oxide, also known as ferric oxide (Fe₂O₃), consists of iron and oxygen atoms arranged in a repeating pattern. In this structure, each iron atom is surrounded by six oxygen atoms in an octahedral shape, and each oxygen atom is connected to two iron atoms. This creates a lattice structure where iron and oxygen atoms alternate, forming a stable and solid crystalline material. The arrangement allows Iron(III) oxide to have strong bonds, making it a durable compound commonly found in nature as the mineral hematite.
To prepare Iron(III) oxide, you can use a simple method involving the reaction between iron and oxygen. When you heat iron in the presence of oxygen, it reacts to form Iron(III) oxide. This process is straightforward and can be performed in a lab setting.
First, you take a piece of iron and heat it strongly in the presence of oxygen. The iron reacts with the oxygen to produce Iron(III) oxide, which appears as a reddish-brown powder. The chemical equation for this reaction is:
Alternatively, you can prepare Iron(III) oxide by decomposing iron(III) hydroxide. When you heat iron(III) hydroxide, it breaks down to form Iron(III) oxide and water. The chemical equation for this reaction is:
Both methods result in the formation of Iron(III) oxide, commonly used in various applications, including pigments and the steel industry.
| Property | Description |
|---|---|
| Color | Reddish-brown |
| State | Solid |
| Density | 5.24 g/cm³ |
| Melting Point | 1565°C |
| Boiling Point | Decomposes before boiling |
| Solubility in Water | Insoluble |
| Magnetic Properties | Weakly magnetic (paramagnetic) |
| Property | Value |
|---|---|
| CAS Registry Number | 1309-37-1 |
| PubChem Compound ID | 14833 |
| SMILES Identifier | [Fe+3].[Fe+3].[O-2].[O-2].[O-2] |
| EU Number | 215-275-4 |
| Gmelin Number | -81 |
| RTECS Number | NO7400000 |
| MDL Number | MFCD00011008 |
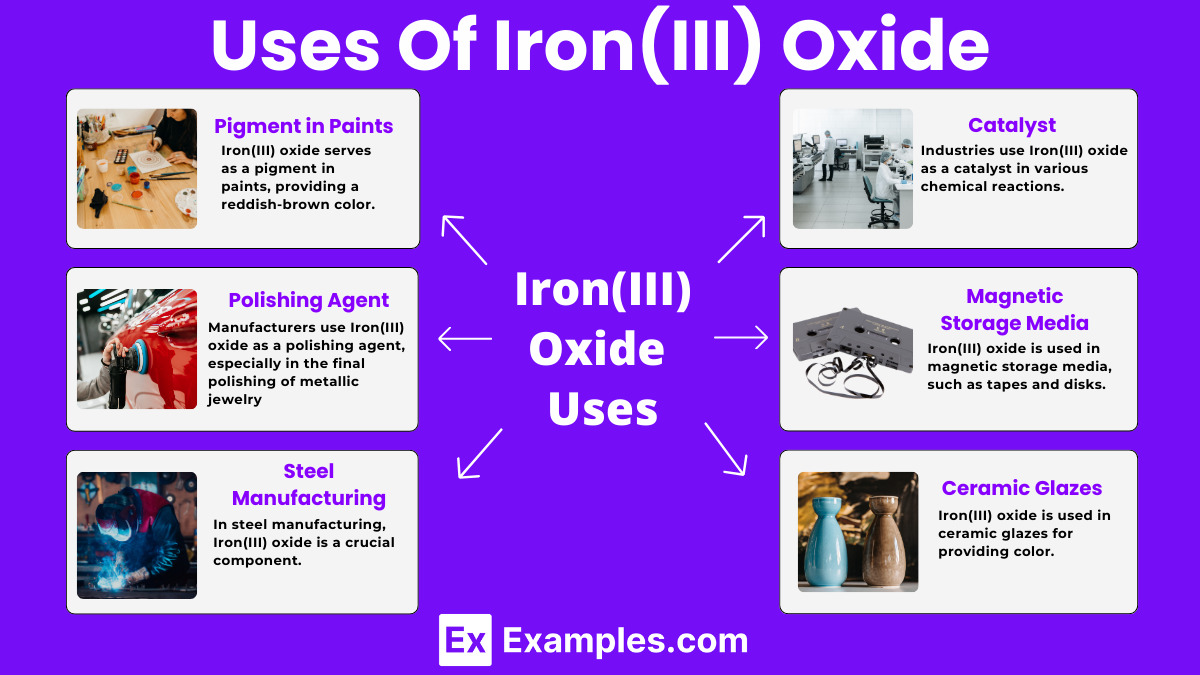
Iron(III) oxide serves as a pigment in paints, providing a reddish-brown color. It is widely used in art and industrial applications.
Manufacturers use Iron(III) oxide as a polishing agent, especially in the final polishing of metallic jewelry and lenses, due to its abrasive properties.
In steel manufacturing, Iron(III) oxide is a crucial component. It is reduced to produce iron, which is then converted into steel.
Iron(III) oxide is used in magnetic storage media, such as tapes and disks, due to its magnetic properties, which help store data effectively.
Industries use Iron(III) oxide as a catalyst in various chemical reactions, such as the production of ammonia in the Haber process.
Iron(III) oxide is used in ceramic glazes, providing color and helping to create different finishes on pottery and tiles.
Iron(III) oxide has the formula Fe₂O₃ because it consists of iron in the +3 oxidation state combined with oxygen atoms.
Iron oxide is also known as ferric oxide or hematite in its natural mineral form.
Yes, rust primarily consists of iron(III) oxide (Fe₂O₃), formed when iron reacts with oxygen and moisture.
Iron oxide can cause skin, eye, and respiratory irritation, but it is generally considered low in toxicity with proper handling.
To make iron oxide rust, expose iron to oxygen and moisture, allowing it to undergo oxidation.
Iron oxide refers to compounds of iron and oxygen, while ferric oxide specifically denotes iron(III) oxide (Fe₂O₃).
Yes, vinegar, containing acetic acid, can dissolve iron oxide by reacting with it and breaking it down.
Iron(III) oxide has ionic bonds between iron cations (Fe³⁺) and oxygen anions (O²⁻).
No, Iron(III) oxide is insoluble in water due to its strong ionic bonds and crystalline structure.
Fe₂O₃ is considered polar due to the difference in electronegativity between iron and oxygen, creating an uneven charge distribution.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary chemical formula for rust, a common form of iron oxide?
FeO
Fe₂O₃
Fe₃O₄
FeO₂
Which type of iron oxide is known as magnetite?
FeO
Fe₂O₃
Fe₃O₄
FeO₂
What color is typically associated with iron(III) oxide?
Black
Red
Green
Blue
Which process is primarily responsible for the formation of iron oxide?
Condensation
Oxidation
Reduction
Precipitation
What is the name of the iron oxide that is commonly used as a pigment in paints and dyes?
Hematite
Magnetite
Iron(III) chloride
Iron(II) sulfate
Which type of iron oxide has both ferrous and ferric iron?
FeO
Fe₂O₃
Fe₃O₄
FeO₂
What is the role of iron oxide in the Earth's crust?
It acts as a catalyst
It contributes to soil fertility
It forms minerals and rocks
It causes water pollution
How does iron oxide affect the color of soil?
It makes soil black
It makes soil red or yellow
It makes soil green
It makes soil white
Which of the following is NOT a common use of iron oxide?
As a pigment in cosmetics
In the manufacture of steel
In magnetic recording media
As a food preservative
What is the process of removing iron oxide from a material called?
Sedimentation
Smelting
Deoxidation
Fermentation
Before you leave, take our quick quiz to enhance your learning!

