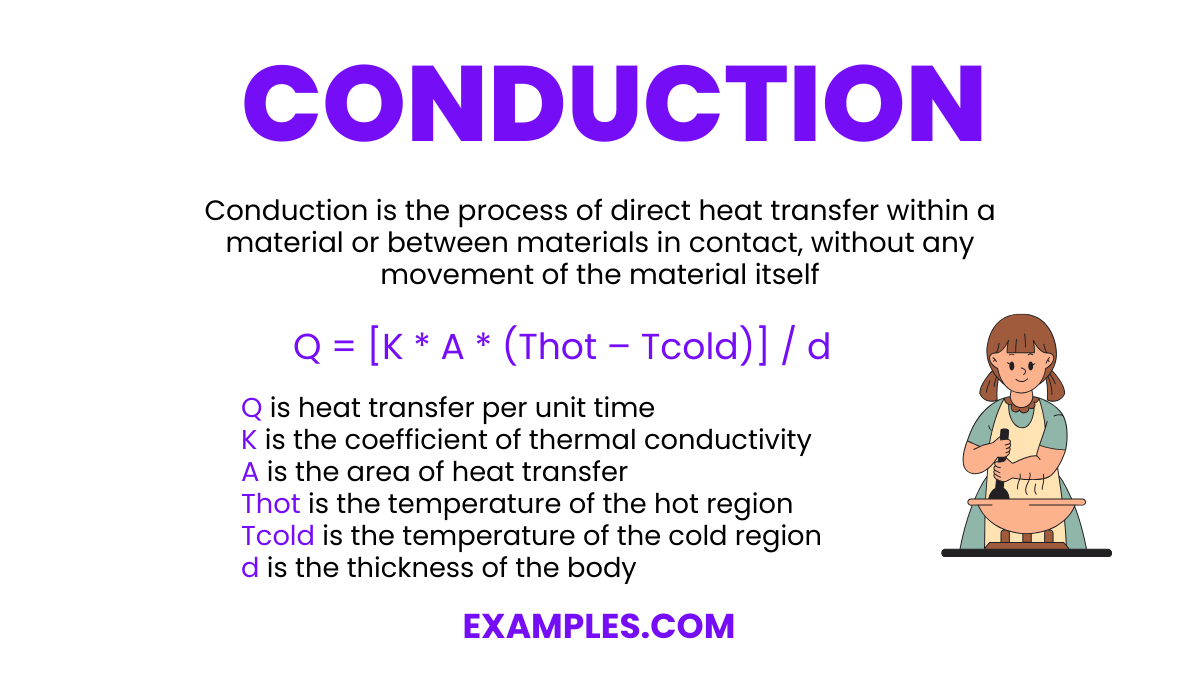
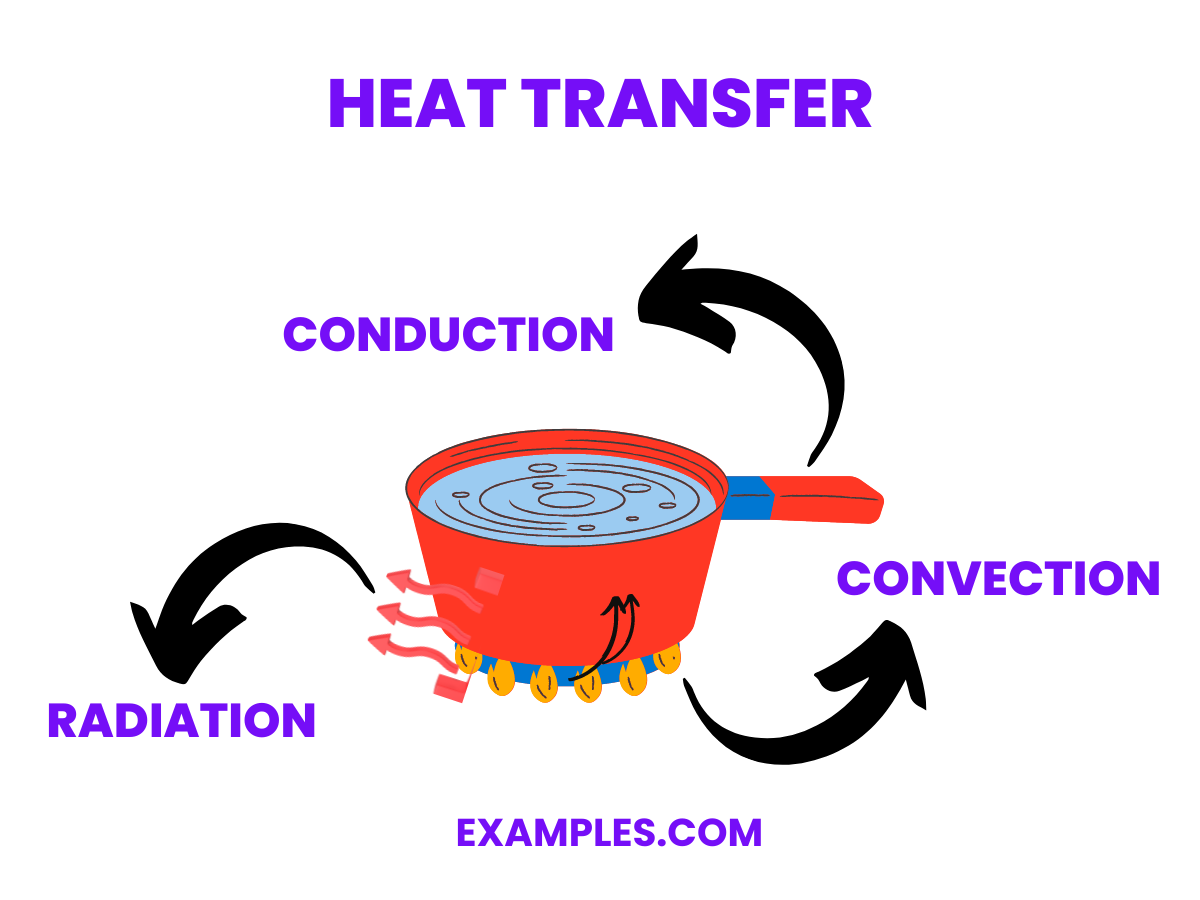
Which of the following best describes thermal conduction?
The transfer of heat through a fluid by convection currents
The transfer of heat through electromagnetic waves
The transfer of heat through direct contact between molecules
The transfer of heat through empty space