What is Raman scattering primarily used to study?
Magnetic properties of materials
Vibrational modes of molecules
Electronic energy levels in atoms
Nuclear reactions

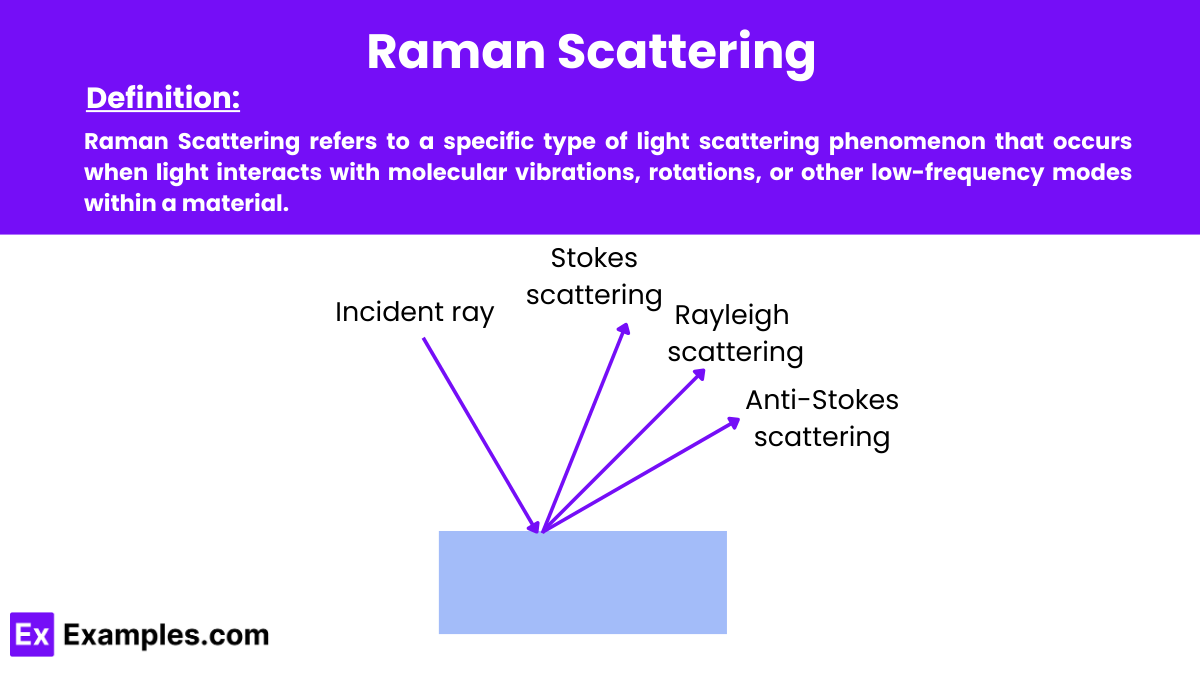
Raman Scattering, also known as Raman Effect, is a phenomenon in physics where light scatters inelastically off molecules, causing the scattered light to shift in energy and wavelength. This scattering occurs when photons interact with molecular vibrations, rotations, or other low-frequency modes in a substance. The change in energy and wavelength of the photons provides insights into the molecular structure and properties of the material. This physical process is governed by the fundamental laws of physics.
A Raman Spectrometer is an analytical instrument used to observe Raman Scattering and measure the Raman spectra of various materials. It primarily functions by shining a monochromatic light source, such as a laser, onto a sample. The light interacts with the molecules in the sample, resulting in the energy of the light being shifted due to vibrational, rotational, and other low-frequency modes within the molecules.
Raman Spectra represent the specific pattern of scattered light produced when light interacts with the molecules of a substance through Raman Scattering. This spectrum displays the intensity of scattered light as a function of its frequency shift relative to the incident light.
When a light source, typically a laser, illuminates a sample, most photons scatter elastically without a change in energy, known as Rayleigh scattering. However, a small fraction of these photons undergo inelastic scattering, gaining or losing energy by interacting with the molecular vibrations of the sample. This change in energy results in a shift in the wavelength of the scattered light, which is captured in the Raman spectra.
Degrees of freedom in physics refer to the independent parameters that define the state of a physical system. These parameters determine the number of independent movements a system can make, encompassing both translational and rotational movements.
In the context of mechanics, degrees of freedom are the individual motions (translations and rotations) that a physical body can undergo in three-dimensional space. For example, a free particle moving in space has three translational degrees of freedom, corresponding to movement along the X, Y, and Z axes. If the particle can also rotate, it may have additional rotational degrees of freedom.
Raman Spectroscopy is a spectroscope technique that measures the Raman spectra of molecules to provide insights into their chemical structures, compositions, and interactions. The technique involves illuminating a sample with a laser light, which interacts with the molecular vibrations, phonons, or other excitations in the sample, leading to the scattering of the light at different energies.
Raman spectroscopy is distinguished by its ability to provide detailed information about molecular vibrations through the interaction of light with matter. Central to understanding Raman spectroscopy are the concepts of Stokes and Anti-Stokes scattering. These phenomena describe how photons scatter when they interact with molecules, leading to shifts in energy that are characteristic of the molecular structure of the sample.
Stokes scattering occurs when photons in the incident light lose energy to the sample during interaction. This energy loss corresponds to an increase in the vibrational energy of the molecules in the sample.
Anti-Stokes scattering occurs when photons in the incident light gain energy from the sample. This happens when a molecule in an excited vibrational state transfers energy to the photon.
Raman Spectroscopy operates on the principle of Raman scattering, where monochromatic light, typically from a laser, interacts with molecular vibrations, rotations, or other forms of low-frequency modes within a material. As the laser light illuminates the sample, most of the light scatters elastically (Rayleigh scattering), which does not alter the energy of the photons. However, a small fraction of the light scatters inelastically (Raman scattering). Resulting in a shift in energy relative to the incident photons.
This shift in energy provides vital information about the molecular structure of the sample. It occurs because the photons either lose or gain energy by interacting with the vibrational energy states of the molecules in the sample. The amount of energy shift and its direction (higher or lower energy) indicate the specific vibrational modes of the molecules. Which are characteristic of their chemical bonds and molecular interactions.
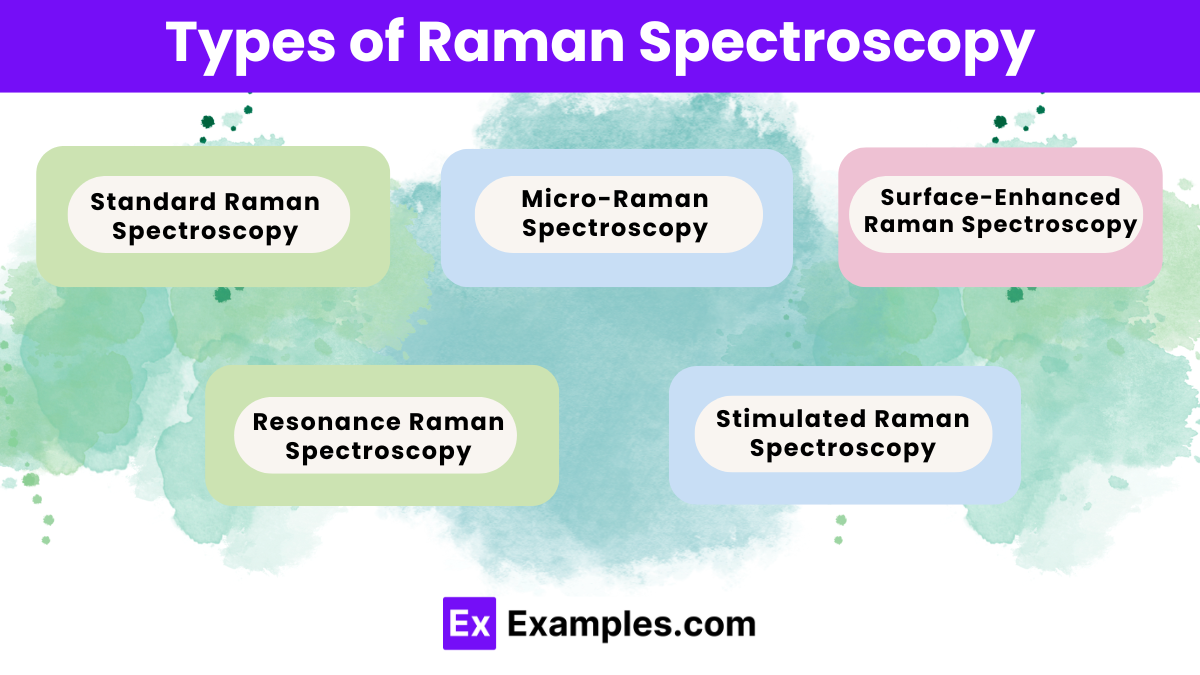
Raman Spectroscopy comes in different types, each suited for specific applications:
Standard Raman Spectroscopy:
Micro-Raman Spectroscopy:
Surface-Enhanced Raman Spectroscopy (SERS):
Resonance Raman Spectroscopy:
Stimulated Raman Spectroscopy:
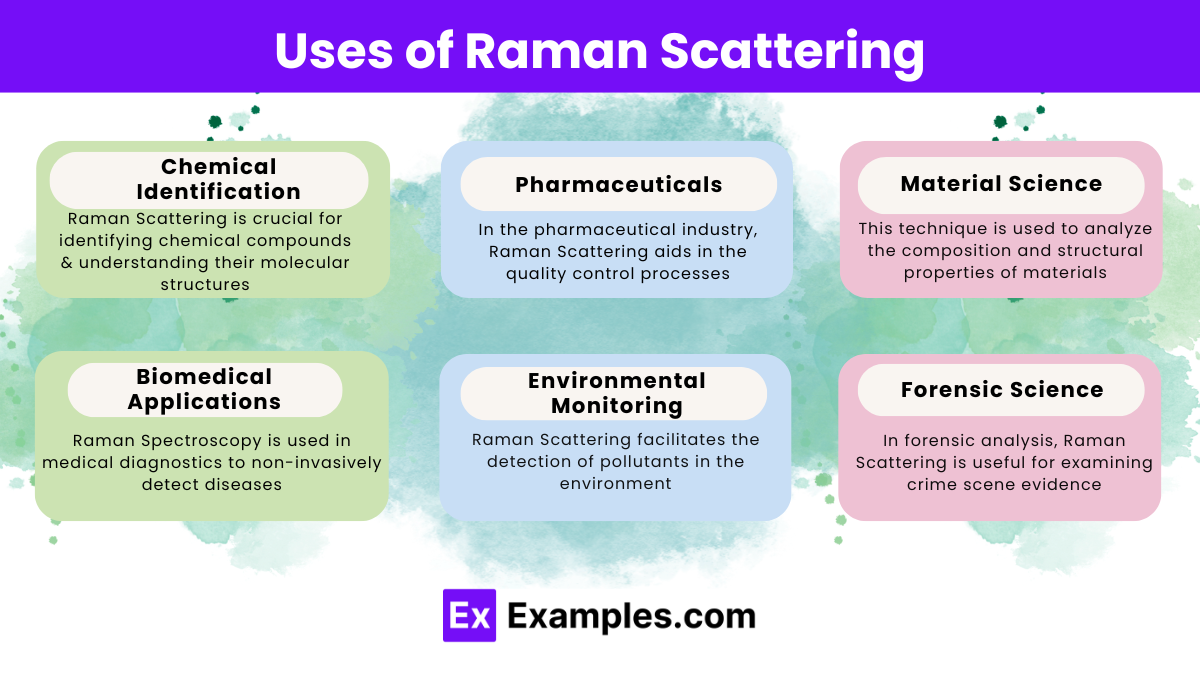
Raman Scattering is a powerful tool utilized across numerous disciplines. Here are some practical examples demonstrate its widespread applications:
Indian physicist C.V. Raman discovered Raman Scattering in 1928, earning him the Nobel Prize in Physics in 1930.
It occurs when incident photons interact with molecules, inducing energy changes and resulting in scatter light with different frequencies.
It provides valuable information about molecular vibrations, structures, and interactions, aiding in various fields like chemistry, materials science, and biomedical research.
Raman Scattering involves energy-level changes without emission of light, while fluorescence involves emission of light after absorb photons.
Raman spectrometers, equipped with lasers, detectors, and spectrometers, are used to analyze scattered light and generate Raman spectra.
It’s a variant of Raman spectroscopy where the excitation wavelength matches the electronic transition energy of the molecule, enhance Raman signals.
It characterizes materials’ chemical compositions, structures, and phases, facilitate research in nanotechnology, polymers, and semiconductors.
Yes, it’s sensitive to subtle changes in molecular vibrations, making it valuable for detecting structural variations and chemical interactions.
Factors include excitation wavelength, sample concentration, laser power, and scattering angle, influence signal strength and spectral quality.
Challenges include fluorescence interference, low signal-to-noise ratios, and complex spectral interpretation, require advanced instrumentation and data analysis techniques.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is Raman scattering primarily used to study?
Magnetic properties of materials
Vibrational modes of molecules
Electronic energy levels in atoms
Nuclear reactions
Which type of light is used in Raman scattering experiments?
X-rays
Ultraviolet light
Visible light
Radio waves
What does the Raman effect involve?
Change in the intensity of light
Change in the wavelength of light due to molecular interactions
Absorption of light by molecules
Emission of photons from a material
In Raman scattering, what is the 'Stokes shift'?
Shift to a higher frequency
Shift to a lower frequency
Shift to a different polarization
No shift occurs
What is the 'anti-Stokes shift' in Raman scattering?
Shift to a lower frequency
Shift to a higher frequency
No shift occurs
Shift in polarization
Which of the following is true about Raman scattering compared to fluorescence?
Raman scattering involves fluorescence emission
Raman scattering provides a broader spectral range
Raman scattering occurs at lower wavelengths
Raman scattering does not involve emission of light
How does the intensity of Raman scattering relate to the concentration of the sample?
It decreases with increasing concentration
It increases with increasing concentration
It remains constant regardless of concentration
It has no relationship with concentration
What is a common application of Raman scattering in material science?
Determining magnetic properties
Analyzing crystal structures
Measuring nuclear stability
Studying chemical reaction rates
Which parameter of Raman scattering is used to identify specific molecular bonds?
Frequency shift
Light polarization
Intensity of scattered light
Incident light wavelength
What does a high Raman scattering cross-section indicate?
Low efficiency of scattering
High efficiency of scattering
No scattering
Decreased sensitivity
Before you leave, take our quick quiz to enhance your learning!

