Which of the following describes a solid's ability to maintain its shape and volume?
Compressibility
Fluidity
Rigidity
Elasticity

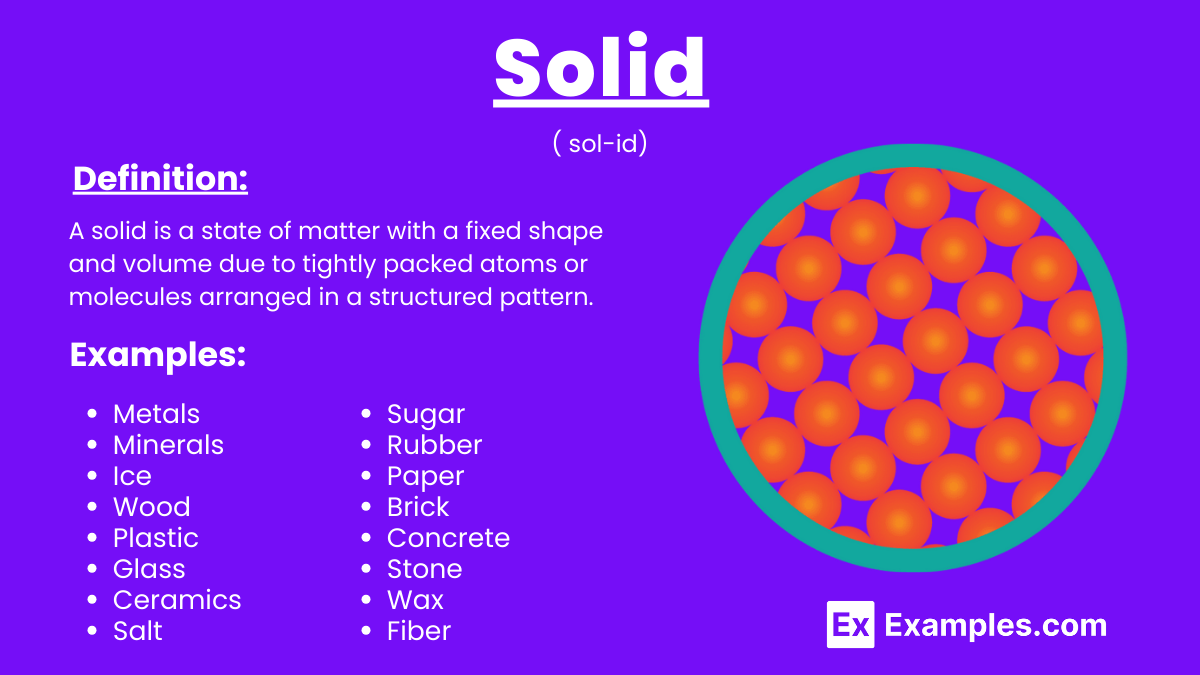
A solid is a state of matter characterized by its definite shape and volume, which results from the close packing of its constituent particles. In a solid, the particles (atoms, molecules, or ions) are arranged in a fixed, orderly structure, allowing only vibrational movement around fixed positions. This arrangement imparts rigidity and resistance to deformation, distinguishing solids from liquids and gases. Common examples include metals, ice, and crystalline structures such as salt. The intermolecular forces in solids are strong enough to hold the particles in place, preventing them from flowing freely like in other states of matter.
A solid is a state of matter with a fixed shape and volume due to tightly packed atoms or molecules arranged in a structured pattern. This arrangement makes solids rigid and incompressible. Examples include ice, rocks, and metals.
Solids can be broadly classified into two main categories: crystalline solids and amorphous solids. Each type has distinct structural and physical properties.
Crystalline solids have a highly ordered and repeating arrangement of atoms, ions, or molecules. This regular pattern extends throughout the entire solid, resulting in a well-defined geometric shape. Crystalline solids can be further divided into several types based on the nature of the bonding between their particles:
Amorphous solids lack a long-range order in their atomic or molecular structure. Unlike crystalline solids, they do not have a regular pattern and do not form well-defined geometric shapes.
Examples: Glass, plastic, rubber
| Type of Solid | Examples | Characteristics |
|---|---|---|
| Ionic Solids | NaCl, CaF₂ | High melting points, brittle, conductive in solution |
| Covalent Network Solids | Diamond, SiC | Extremely hard, very high melting points, poor conductors |
| Metallic Solids | Fe, Cu | Good conductors, malleable, ductile |
| Molecular Solids | Ice, dry ice | Low melting points, soft, poor conductors |
| Amorphous Solids | Glass, plastic, rubber | Lack long-range order, soften over a range |
Solids play a crucial role in various aspects of daily life and industrial applications. Here are some key uses of solids:
Particles in a solid vibrate around fixed positions, maintaining a stable structure and shape.
Common examples include table salt (sodium chloride) and diamonds, both having well-defined geometric structures.
Increasing temperature causes solids to expand slightly and may eventually lead to melting if the temperature is high enough.
The melting point is the temperature at which a solid changes into a liquid.
Hardness measures a solid’s resistance to deformation or scratching. Diamond is the hardest known natural material.
Density depends on mass and volume. Metals typically have high density, while materials like wood have lower density.
A lattice structure is a repeated three-dimensional arrangement of atoms, ions, or molecules in a crystalline solid.
Metals are good conductors of electricity due to free-moving electrons, while nonmetals and insulators do not conduct well.
Elasticity is the ability of a solid to return to its original shape after being deformed, such as stretching or compressing.
Plasticity is the property that allows a solid to permanently deform without breaking when a force is applied.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which of the following describes a solid's ability to maintain its shape and volume?
Compressibility
Fluidity
Rigidity
Elasticity
What type of bond is typically found in crystalline solids?
Ionic bond
Metallic bond
Covalent bond
Hydrogen bond
Which of the following is a characteristic of an amorphous solid?
Well-defined melting point
Regular atomic arrangement
Lack of long-range order
High electrical conductivity
In which type of solid do atoms or molecules occupy fixed positions in a regular array?
Metallic solids
Molecular solids
Ionic solids
Network solids
Which property is most likely to be high in metallic solids?
Electrical conductivity
Melting point
Solubility in water
Brittle behavior
What type of solid is quartz classified as?
Molecular solid
Ionic solid
Network solid
Metallic solid
Which characteristic best describes a crystalline solid's structure?
Disordered
Random
Repeating pattern
Amorphous
What is the primary force that holds together the layers in a layered solid like graphite?
Ionic bonds
Covalent bonds
Van der Waals forces
Hydrogen bonds
Which of the following properties is generally low in amorphous solids?
Melting point
Solubility
Density
Thermal conductivity
What type of solid is table salt (sodium chloride)?
Metallic solid
Molecular solid
Ionic solid
Network solid
Before you leave, take our quick quiz to enhance your learning!

