What is the formula for Coulomb\'s law?
F = k(q1q2)/r²
F = k(q1+q2)/r²
F = k(q1q2)/r
F = k(q1-q2)/r²

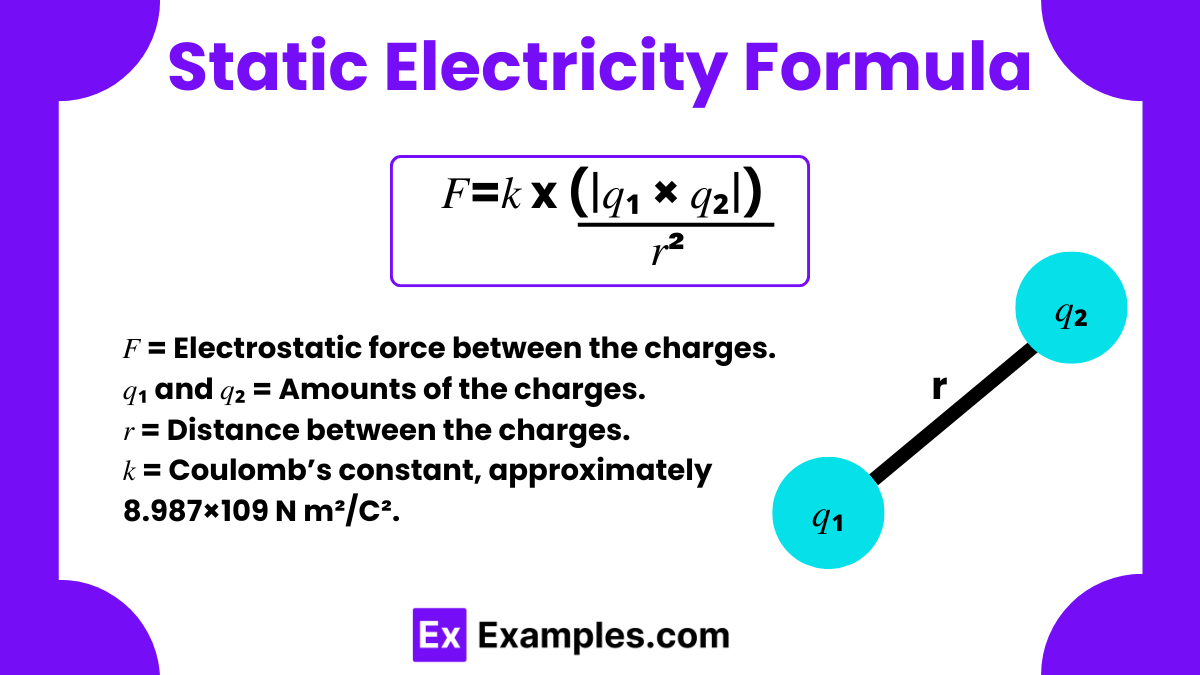
Static electricity refers to the electric charge that builds up on the surface of objects. The static electricity formula used to calculate the force between two charged objects due to static electricity is derived from Coulomb’s Law. This law was discovered by Charles-Augustin de Coulomb in 1785. It states that the force between two point charges is directly proportional to the product of the magnitudes of the charges and inversely proportional to the square of the distance between them.
The formula is expressed as:
First, we establish that the force 𝐹F between two point charges is proportional to the product of the magnitudes of the charges (𝑞1 and 𝑞2) and inversely proportional to the square of the distance (𝑟r) between them:
To convert the proportionality into an equation, we introduce Coulomb’s constant 𝑘k, which has a value of approximately 8.987×1098.987×109 N·m²/C². This constant aligns the units and provides the correct scaling factor based on experimental evidence:
Problem: Two small spheres are charged with +3 microcoulombs and -5 microcoulombs respectively. They are 0.5 meters apart in a vacuum. Calculate the force between them using Coulomb’s Law.
Solution:
Using the formula, 𝐹=𝑘 ( (∣𝑞₁×𝑞₂∣) / 𝑟²)
𝐹=8.987×10⁹× ( (∣3×10⁻⁶ × −5×10⁻⁶) / 0.5²)
𝐹=8.987×10⁹ ×( (15×10⁻¹² ) / 0.25)
𝐹=8.987×10⁹×60×10⁻¹²
𝐹=539.22 N
The force is attractive since the charges are opposite.
Problem: What is the Electrostatic force between two charged objects with charges of +10 Microcoulombs each, placed 2 meters apart?
Solution:
Using the formula, 𝐹=𝑘( (∣𝑞₁×𝑞₂∣) / 𝑟²)
𝐹=8.987×10⁹×( ((10×10⁻⁶)×(10×10⁻⁶)) / 2² )
𝐹=8.987×10⁹×( (100×10⁻¹²) / 4)
𝐹=8.987×10⁹×25×10⁻¹²
𝐹=224.675 N
The force is repulsive as both charges are positive.
Problem: Calculate the force exerted between a charge of +2 Microcoulombs and another charge of +6 Microcoulombs, separated by a distance of 3 meters.
Solution:
Using the formula, 𝐹=𝑘 ( (∣𝑞₁×𝑞₂∣) / 𝑟²)
𝐹=8.987×10⁹ × ( (2×10⁻⁶×6×10⁻⁶) / 3²)
𝐹=8.987×10⁹ × ((12×10⁻¹²) / 9))
𝐹=8.987×10⁹ × 1.333×10⁻¹²
𝐹=11.983 N
The force is also repulsive, with both charges being positive.
Static energy can’t calculated. We can calculate static force using Coulomb’s Law.
The electrostatic equation refers to Coulomb’s Law: 𝐹= (1/4πε0) x (∣𝑞₁×𝑞₂∣ / 𝑟²), which calculates the force between two charges.
You can feel static electricity at voltages as low as 3,000 volts, typical in everyday static discharges.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the formula for Coulomb\'s law?
F = k(q1q2)/r²
F = k(q1+q2)/r²
F = k(q1q2)/r
F = k(q1-q2)/r²
What does the constant 'k' represent in Coulomb's law?
Gravitational constant
Coulomb's constant
Planck's constant
Boltzmann's constant
What is the unit of electric charge in the International System of Units (SI)?
Volt
Coulomb
Ampere
Newton
What is the formula for electric field (E) due to a point charge (q)?
E = kq/r²
E = kq/r
E = kq²/r²
E = kq²/r
What is the value of the elementary charge (e)?
1.6 × 10⁻¹⁹ C
1.6 × 10⁻¹⁸ C
1.6 × 10⁻²⁰ C
1.6 × 10⁻¹⁷ C
How is electric potential (V) related to electric field (E) and distance (d)?
V = Ed
V = E/d
V = E²d
V = E/d²
What is the unit of electric potential?
Newton
Joule
Volt
Watt
What is the formula for the potential energy (U) of a charge (q) in an electric field (E)?
U = qE
U = qEd
U = qE/d
U = qE²
How is capacitance (C) defined?
C = QV
C = QV
C = QV²
C = Q/V²
What does the principle of superposition state in electrostatics?
The total force is the sum of individual forces
The total force is the product of individual forces
The total force is the difference of individual forces
The total force is the ratio of individual forces
Before you leave, take our quick quiz to enhance your learning!

