What is the First Law of Thermodynamics?
Energy cannot be created or destroyed, only transformed
The entropy of a system always increases
For every action, there is an equal and opposite reaction
Energy flows from cold to hot

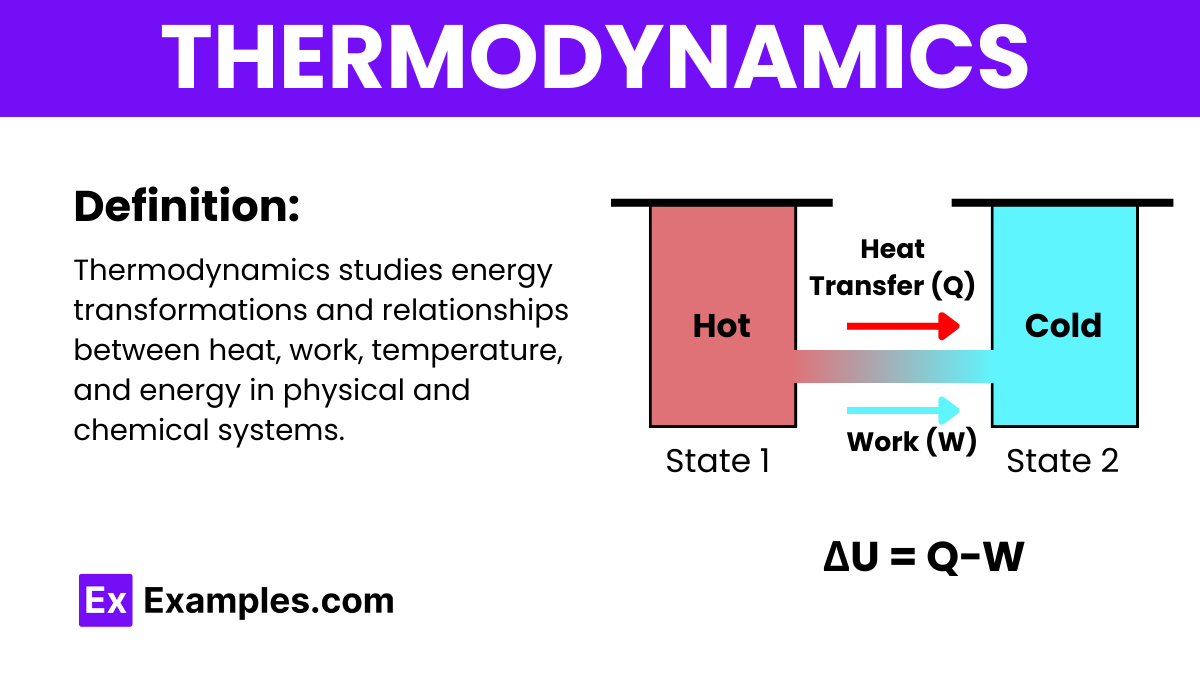
Thermodynamics is a branch of physics that studies the relationships and conversions between heat and other forms of energy. It examines how energy transformations are governed by certain laws, influencing various physical processes and systems.
A fundamental and commonly used formula in thermodynamics is the First Law of Thermodynamics, which is expressed as:
Where:
This formula encapsulates the principle of energy conservation within a thermodynamic system, indicating that any increase in the internal energy (ΔU) of the system is the result of the net heat added to the system (Q) minus the work done by the system on its surroundings (W).

Classical thermodynamics focuses on the macroscopic behavior of energy and materials without considering atomic details. It is based on the laws of thermodynamics, which define relationships between heat, work, temperature, and energy in systems.
Statistical thermodynamics bridges microscopic atomic and molecular behavior with macroscopic observations using statistical methods to explain thermodynamic phenomena, focusing on the behavior of systems at the particle level.
Chemical thermodynamics applies thermodynamic principles to chemical reactions and energy changes, emphasizing the study of reaction energies and equilibrium.
This branch deals with systems at thermodynamic equilibrium, where macroscopic properties do not change over time. It involves the study of energy distributions and phase transitions under equilibrium conditions.
Non-equilibrium thermodynamics examines systems away from equilibrium, which is essential for understanding real-world systems where energy and matter transfers are unbalanced.
Thermodynamics is a critical discipline within physics and engineering that has wide-reaching implications across numerous fields. Here’s why thermodynamics is so important:
Thermodynamics helps explain natural phenomena at both macroscopic and microscopic levels. It provides insights into the workings of everything from atmospheric dynamics and ocean currents to biological processes and the fundamental operations of the universe.
It is essential for the efficient design, operation, and analysis of systems that convert energy from one form to another, such as engines, power plants, and refrigerators. Thermodynamics enables the optimization of these systems for more sustainable energy use and increased performance.
Thermodynamics plays a pivotal role in environmental engineering and sustainability studies. It helps assess the impact of energy usage on the environment, facilitating the development of technologies that reduce waste and improve energy efficiency.
The principles of thermodynamics are fundamental to developing new materials and technologies, including advances in renewable energy sources, automotive industry improvements, and innovative solutions in electronics and materials science.
In the chemical industry, thermodynamics is crucial for the design and operation of processes that involve chemical reactions, separation processes, and the production of compounds. It helps in predicting reaction outcomes, energy needs, and material properties under various conditions.
By improving the efficiency of thermal systems, thermodynamics directly influences economic efficiency in manufacturing, power generation, heating, and cooling systems. This reduces costs and energy consumption, leading to more economically viable operations.
Thermodynamics is a foundational subject in science and engineering education, enabling students and researchers to apply its concepts to a variety of complex problems and innovations.
In the field of biotechnology and medical sciences, thermodynamics helps in understanding metabolic processes and the physiochemical aspects of body functions, contributing to medical diagnostics, drug design, and other therapeutic practices.
Temperature is a measure of the average kinetic energy of the particles in a substance. It’s fundamental for describing heat transfer between systems and is measured in degrees Celsius (°C), Kelvin (K), or Fahrenheit (°F).
Pressure is the force exerted per unit area by the particles of a substance. It is a critical factor in determining the behavior of gases and liquids and is measured in pascals (Pa) or atmospheres (atm).
Volume is the space occupied by a substance. Changes in volume can significantly affect pressure and temperature of a system, especially in gaseous states.
Internal energy is the total energy contained within a system, including kinetic and potential energies of particles. It changes with heat transfer and work done by or on the system.
Entropy measures the disorder or randomness in a system. It is a central concept in the Second Law of Thermodynamics, indicating that systems tend to move towards more disordered states.
Enthalpy is the total heat content of a system at constant pressure. It is used especially in scenarios involving heat transfer in chemical reactions and phase changes.
Specific heat capacity is the amount of heat required to change the temperature of a unit mass of a substance by one degree Celsius. It varies among different materials and is crucial for thermal management.
Gibbs free energy is a thermodynamic potential that measures the maximum reversible work that may be performed by a thermodynamic system at constant temperature and pressure. It is particularly useful in chemical thermodynamics to predict the direction of chemical reactions.
Helmholtz free energy is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature. It’s valuable for understanding systems that perform work at stable temperatures.
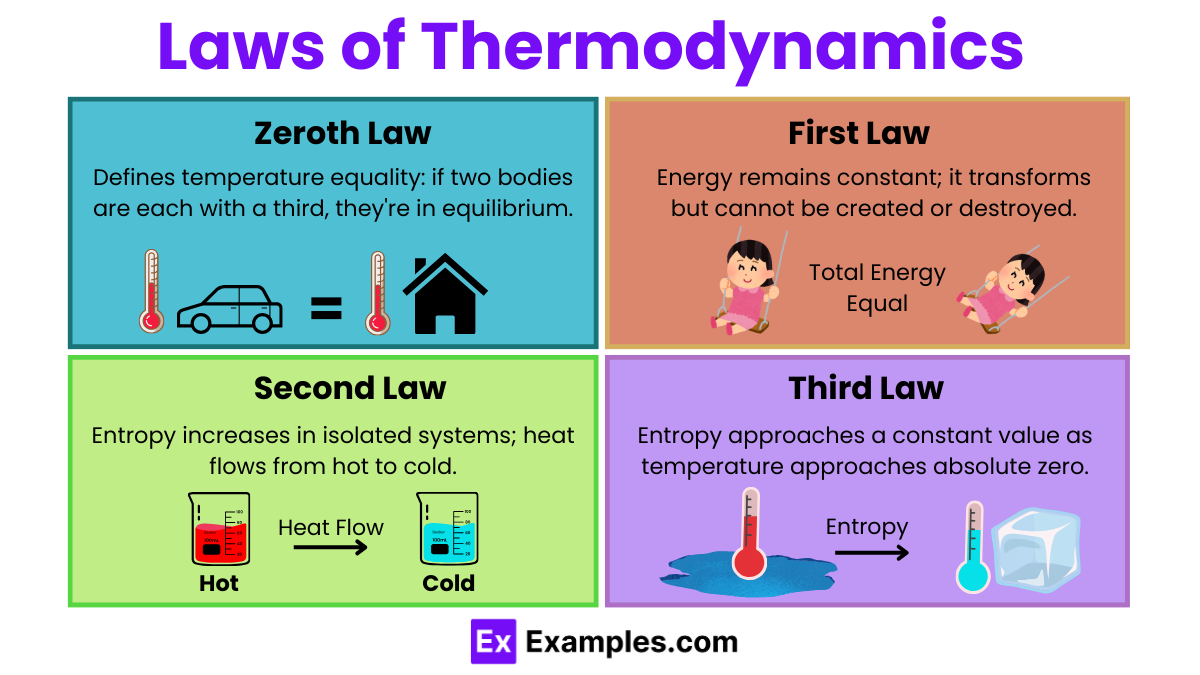
Thermodynamics is governed by several fundamental laws that describe the behavior of energy in various systems. Here’s a detailed look at these laws along with relevant equations and formulas:
Principle: The Zeroth Law establishes a basis for temperature measurement, stating that if two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
Implication: This law allows for the definition of temperature in a practical sense and is the foundation of temperature measurement.
Principle: Energy cannot be created or destroyed in an isolated system. The change in the internal energy of a system is equal to the heat added to the system minus the work done by the system on its surroundings.
Equation: ΔU = Q−W Where:
Principle: The total entropy of an isolated system can never decrease over time. It remains constant if all processes are reversible and increases if they are irreversible. This law also states that heat cannot spontaneously transfer from a colder body to a hotter body.
Equations: For a reversible process, ΔS=∫dQᵣₑᵥ/T Where:
For spontaneous processes in isolated systems, ΔS>0
Principle: As the temperature of a system approaches absolute zero, the entropy of the system approaches a constant minimum.
Implication: This law implies that it is impossible to reach absolute zero in a finite number of operations, and as temperature approaches absolute zero, the system displays less and less energy.
Mathematical Formulation: limₜ→₀S=S₀ Where S₀ is typically zero for perfectly crystalline substances.
| Aspect | Mechanics | Thermodynamics |
|---|---|---|
| Definition | Mechanics is the branch of physics that deals with the motion of bodies under the action of forces. | Thermodynamics is the study of the relationships between heat, work, temperature, and energy in a system. |
| Focus | Focuses on the forces and their effects on motion. | Focuses on energy transformations and the state properties of systems. |
| Laws | Newton’s laws of motion and principles of conservation of momentum are central. | Laws of thermodynamics, which include energy conservation, entropy, and absolute temperatures, are central. |
| Applications | Applied in studying objects from sub-atomic particles to astronomical objects in motion. | Applied in understanding and designing heat engines, refrigerators, and all manners of thermal systems. |
| Quantities | Deals primarily with force, mass, acceleration, displacement, and velocity. | Deals with temperature, heat, internal energy, entropy, and enthalpy. |
| Equations | Key equations include F=ma (Newton’s second law) and equations of motion. | Key equations include the first and second laws of thermodynamics (ΔU=Q−W, ΔS≥0). |
| Nature of Study | Often involves deterministic approaches where the outcomes are predictable based on initial conditions. | Often involves statistical approaches, especially in systems involving large numbers of particles. |
| Practical Examples | Design of structures, vehicles, machines, and studying planetary motions. | Design of HVAC systems, power plants, engines, and understanding biological and chemical processes. |
Thermodynamics revolves around the study of energy transfers and transformations in physical and chemical processes, emphasizing heat, work, temperature, and entropy.
The first law states that energy in a closed system is constant; it cannot be created or destroyed, only converted from one form to another.
There is no officially recognized 5th law of thermodynamics; the discipline traditionally includes only four fundamental laws.
Yes, the human body obeys all thermodynamic laws, including energy conservation and entropy changes, through metabolic and physiological processes.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the First Law of Thermodynamics?
Energy cannot be created or destroyed, only transformed
The entropy of a system always increases
For every action, there is an equal and opposite reaction
Energy flows from cold to hot
What does the Second Law of Thermodynamics state about entropy?
Entropy of an isolated system always decreases
Entropy of an isolated system always increases
Entropy of an isolated system remains constant
Entropy is not applicable to isolated systems
In thermodynamics, what is an isothermal process?
A process at constant pressure
A process at constant volume
A process at constant temperature
A process at constant entropy
What is the significance of the Zeroth Law of Thermodynamics?
It defines temperature
It explains entropy
It establishes energy conservation
It describes heat transfer
In an adiabatic process, what happens to the heat exchange with the surroundings?
Heat is absorbed
Heat is released
No heat exchange occurs
Heat exchange depends on pressure
What does the Third Law of Thermodynamics state about absolute zero?
It can be reached under certain conditions
It cannot be reached in a finite number of steps
It has no impact on entropy
It defines the behavior of perfect gases
Which thermodynamic quantity measures the disorder of a system?
Enthalpy
Internal energy
Entropy
Free energy
What is the formula for the change in internal energy (ΔU) in a closed system according to the First Law of Thermodynamics?
ΔU = Q - W
ΔU = Q + W
ΔU = Q
ΔU = W
In a thermodynamic cycle, what is the net change in internal energy after one complete cycle?
Zero
Equal to the heat added
Equal to the work done
Equal to the entropy change
What is enthalpy (H) defined as in thermodynamics?
H = U - PV
H = U + PV
H = Q - W
H = Q + W
Before you leave, take our quick quiz to enhance your learning!

