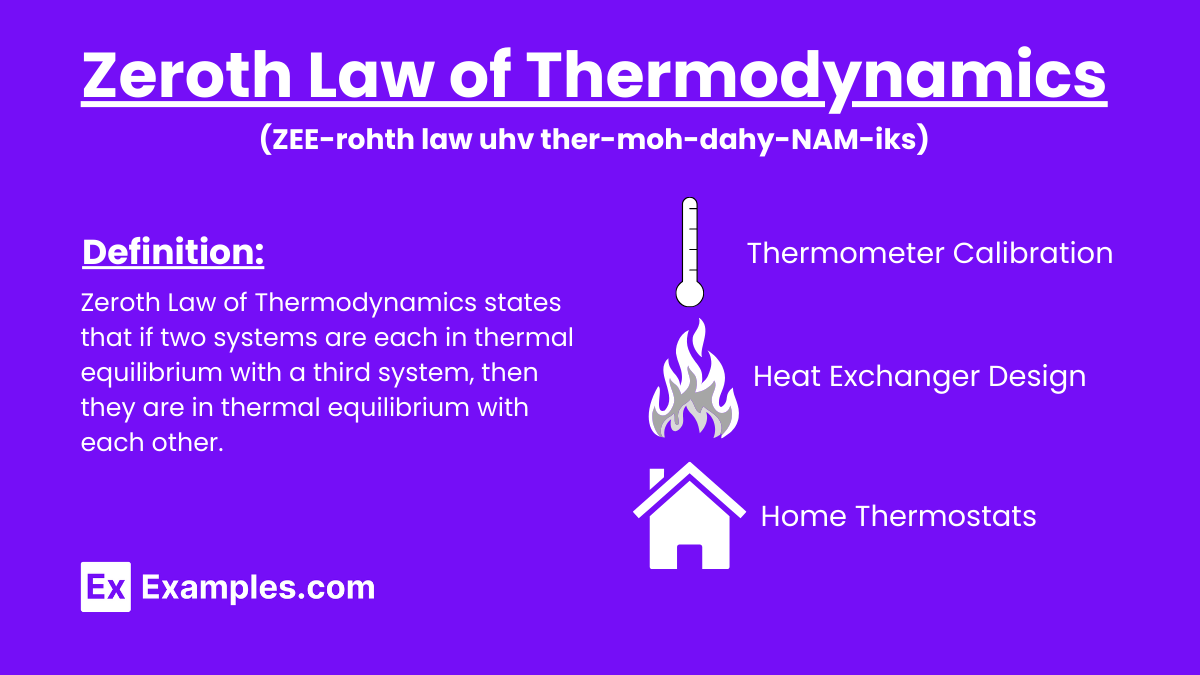
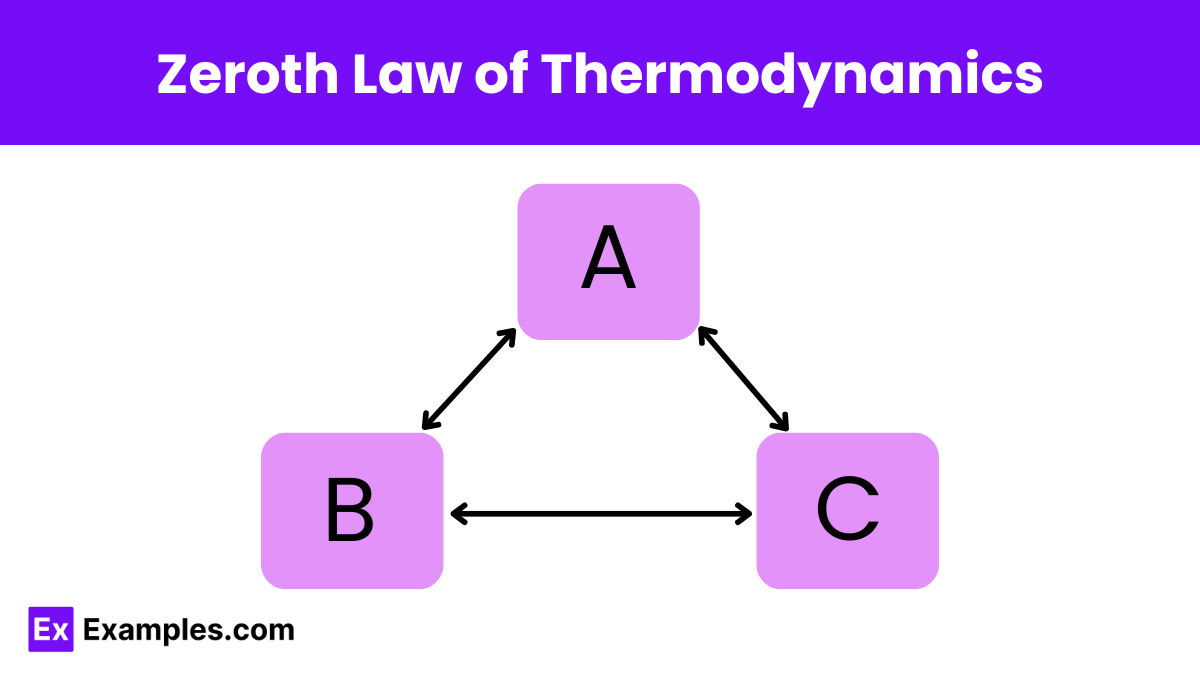
What does the Zeroth Law of Thermodynamics state about thermal equilibrium?
If two systems are each in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
The temperature of a system always increases when it is in contact with another system.
Two systems in thermal contact will always exchange heat until they reach the same temperature.
Temperature is not a measurable property of a system.