What is the atomic number of Copper?
27
29
31
33
Discover the versatile world of Copper, a fundamental metal pivotal in various industries, including electrical, construction, and jewelry. This comprehensive guide delves into Copper’s properties, applications, and historical significance, offering insightful examples of its utility and importance. Uncover the benefits of Copper, from its superior conductivity to its antimicrobial features, and explore how this durable, recyclable metal continues to shape technological advancements and sustainable solutions. Join us as we navigate the multifaceted applications of Copper, revealing its enduring relevance in modern society.
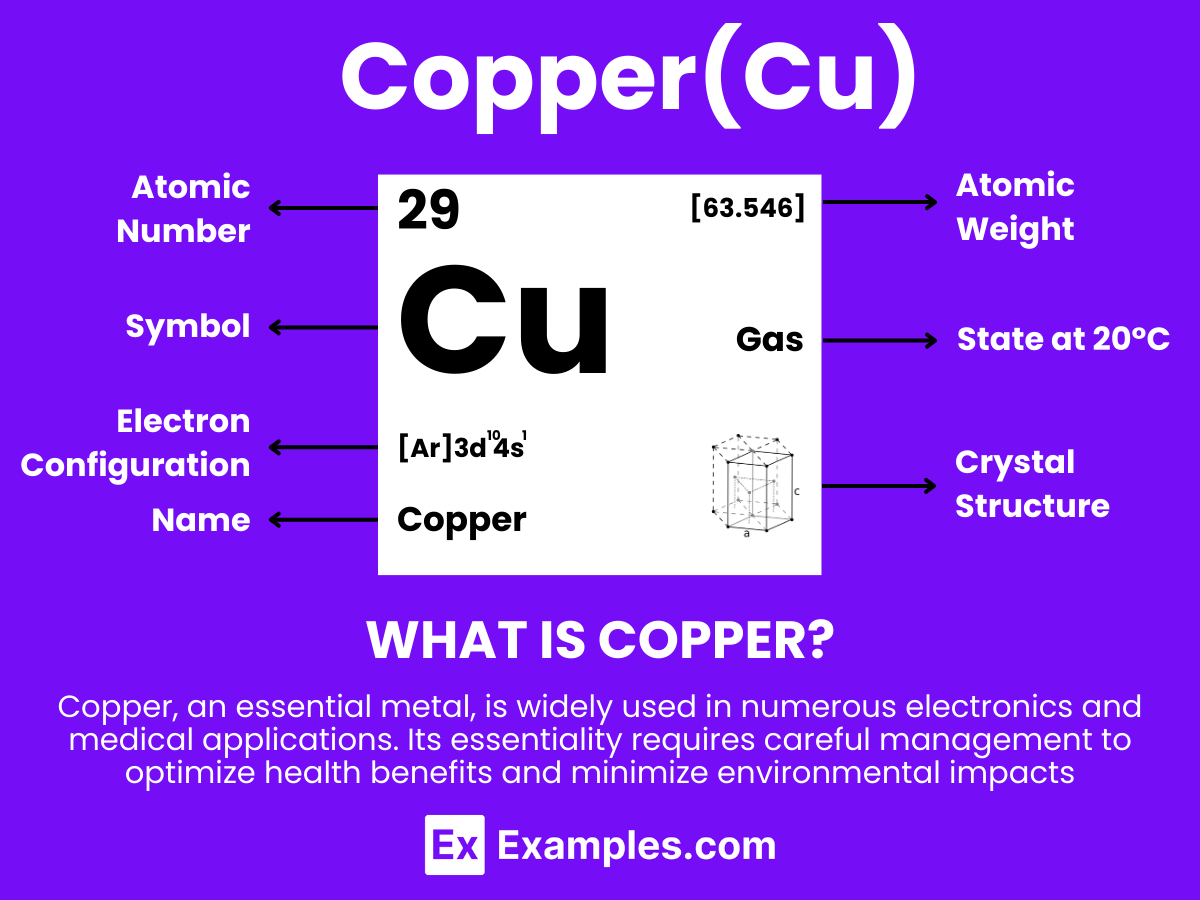
Copper is a chemical element with the symbol Cu and atomic number 29. It is a transition metal known for its reddish-brown appearance and excellent conductivity. Copper does not tarnish easily when exposed to air, making it highly resistant to corrosion and perfect for electrical wiring. Historically, Copper has been used in coinage and alloys such as brass and bronze, and today, it finds applications in electrical equipment, plumbing, and renewable energy technologies. For teachers, Copper serves as an outstanding example to discuss the properties of elements, their position in the periodic table, and their extensive applications in daily life.
Formula: Cu
Composition: A single copper atom.
Bond Type: Copper forms metallic bonds in its metallic state and can form covalent or ionic bonds in its compounds, with one or two valence electrons in the outer shell.
Molecular Structure: Exists mainly in one form as metallic copper, which is highly conductive and malleable.
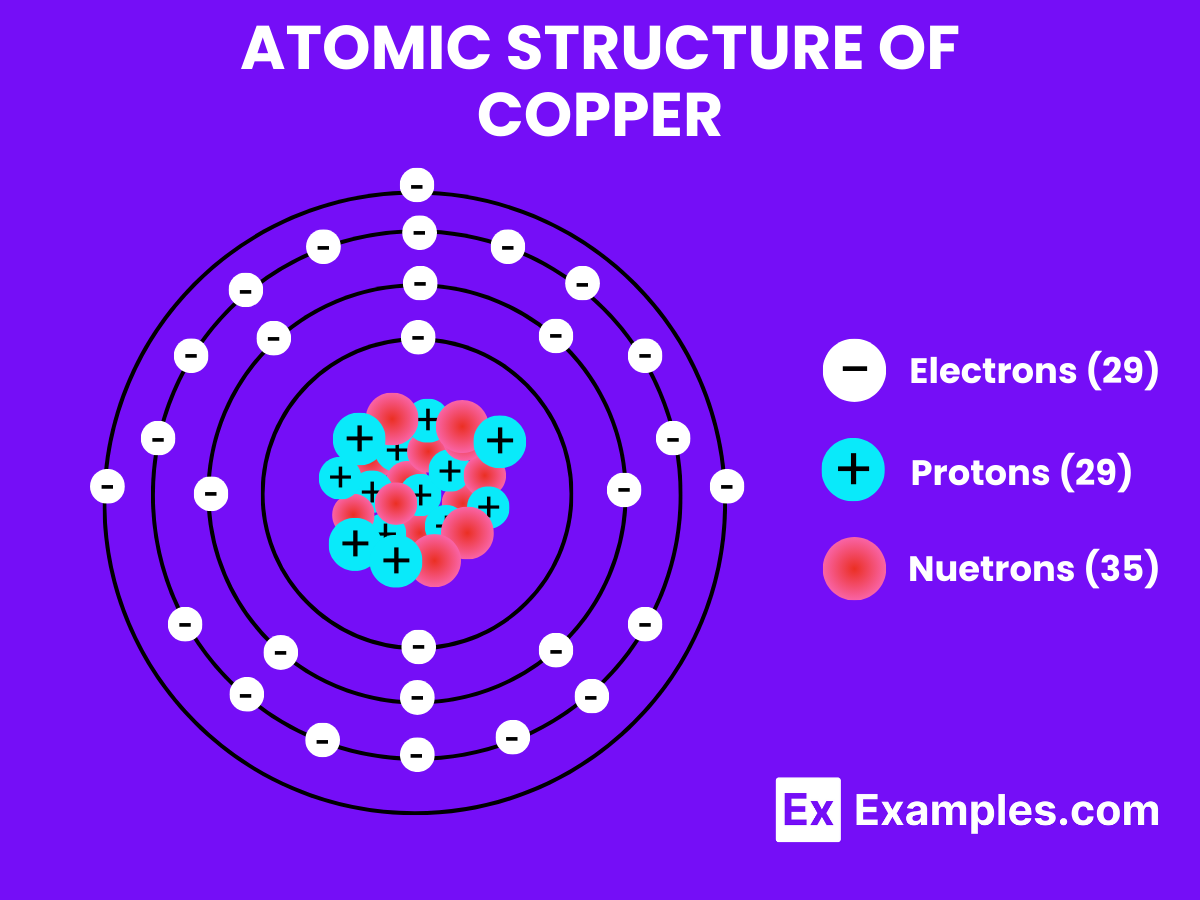
Electron Configuration: 29 electrons, with the configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s¹.
Significance: Essential for electrical wiring, plumbing, and as a component in various alloys like brass and bronze.
Role in Chemistry: Key in the study of electrical conductivity and the formation of alloys, as well as in bioinorganic chemistry due to its biological importance.
| Property | Value |
|---|---|
| Appearance | Reddish-brown, metallic luster |
| Density | 8.96 g/cm³ |
| Melting Point | 1084.62°C (1984.32°F) |
| Boiling Point | 2562°C (4643.6°F) |
| State at Room Temperature | Solid |
| Thermal Conductivity | 401 W/(m·K) |
| Electrical Conductivity | 5.96 × 10⁷ S/m (at 20°C) |
| Heat Capacity | 0.385 J/(g·K) |
Copper, with the chemical symbol Cu and atomic number 29, showcases a variety of chemical properties that contribute to its wide range of applications:
| Property | Value |
|---|---|
| Melting Point | 1,083°C (1,981°F) |
| Boiling Point | 2,567°C (4,653°F) |
| Specific Heat Capacity | 0.385 J/g·K |
| Thermal Conductivity | 401 W/m·K |
| Heat of Fusion | 13.26 kJ/mol |
| Heat of Vaporization | 300.4 kJ/mol |
| Property | Value |
|---|---|
| Density | 8.96 g/cm³ |
| Young’s Modulus | 110-128 GPa |
| Tensile Strength | 210-220 MPa |
| Hardness (Brinell) | 874 MPa |
| Elastic Modulus | 117 GPa |
| Poisson’s Ratio | 0.34 |
| Property | Value |
|---|---|
| Electrical Conductivity | 5.96×10⁷ S/m |
| Electrical Resistivity | 1.68×10⁻⁸ Ω·m |
| Magnetic Susceptibility | -9.6×10⁻⁶ cm³/mol (diamagnetic) |
| Property | Value |
|---|---|
| Natural Isotopes | Cu-63 (69.17%), Cu-65 (30.83%) |
| Neutron Cross Section | 3.78 barns (for Cu-63) |
| Neutron Mass Absorption | 0.0 |
The preparation of copper involves several key steps to extract and refine the metal from its ores, primarily from chalcopyrite (CuFeS₂), the most abundant copper ore. The process encompasses:
| Isotope | Natural Abundance | Half-Life | Mode of Decay |
|---|---|---|---|
| Cu-63 | 69.15% | Stable | N/A |
| Cu-65 | 30.85% | Stable | N/A |
| Cu-64 | Trace | 12.7 hours | β+ decay to Ni-64, β- decay to Zn-64, Electron capture |
| Cu-67 | Synthetic | 61.83 hours | β- decay to Zn-67 |
Copper has two stable isotopes, Cu-63 and Cu-65, which are found naturally in the environment. The other isotopes, like Cu-64 and Cu-67, are radioactive and produced synthetically in laboratories. These isotopes have applications in medicine and scientific research.
Copper is a versatile metal with a wide range of applications, thanks to its excellent electrical and thermal conductivity, corrosion resistance, and malleability. Some of the most significant uses of copper include:
| Country | Mine Production 2016 (Metric Tons)* | % of World Mine Production | Demonstrated Reserves 2016 (Metric Tons)* | % of World Demonstrated Reserves |
|---|---|---|---|---|
| Chile | 5,500,000 | 26.9% | 170,000,000 | 28.0% |
| Peru | 2,350,000 | 11.5% | 81,000,000 | 13.3% |
| China | 1,740,000 | 8.5% | 30,000,000 | 4.9% |
| United States | 1,410,000 | 6.9% | 35,000,000 | 5.7% |
| Congo (DRC) | 1,030,000 | 5.0% | 20,000,000 | 3.3% |
| Australia | 970,000 | 4.7% | 88,000,000 | 14.5% |
| Russia | 710,000 | 3.5% | 30,000,000 | 4.9% |
| Zambia | 708,000 | 3.4% | 20,000,000 | 3.3% |
| Canada | 696,000 | 3.4% | 11,000,000 | 1.8% |
| Mexico | 575,000 | 2.8% | 38,000,000 | 6.2% |
The production of copper involves a series of processes that extract the metal from its ores and refine it to a usable purity level. The main steps include:
Copper’s properties, such as high conductivity, ductility, and corrosion resistance, make it suitable for a wide range of applications:
This article provided a comprehensive exploration of copper, highlighting its extraction, refining process, isotopes, and wide-ranging applications. Copper’s remarkable properties make it indispensable in electrical wiring, construction, renewable energy, and more. Understanding copper’s production and multifaceted uses showcases its vital role in advancing technology and supporting modern infrastructure, emphasizing its significance in our daily lives and future developments.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Copper?
27
29
31
33
Copper is primarily used in electrical wiring because it is:
Magnetic
An excellent conductor of electricity
Highly reactive
Transparent
Which alloy is formed by combining copper and tin?
Steel
Brass
Bronze
Pewter
Copper can be found in which type of ore?
Hematite
Chalcopyrite
Galena
Bauxite
Which property does copper have that makes it useful for cookware?
Low melting point
High corrosion resistance
High thermal conductivity
High electrical resistance
Copper roofing materials are valued because they:
Increase the structural strength of buildings
Are inexpensive
Develop a protective patina
Are easy to paint
Which vitamin is required for the body to properly utilize copper?
Vitamin C
Vitamin D
Vitamin B12
Vitamin A
What is the primary effect of copper deficiency in humans?
Increased risk of infection
Memory loss
Anemia
Weight gain
The Statue of Liberty's green color is due to:
Paint
Copper oxidation
Use of green stone
Algae
How is copper recycled?
Melting down and reforming into new products
Dissolving in acids
Compressing into pellets
Chemical separation
Before you leave, take our quick quiz to enhance your learning!

