What does Boyle's Law state about the relationship between pressure and volume of a gas at constant temperature?
Directly proportional
Inversely proportional
No relationship
Linearly proportional

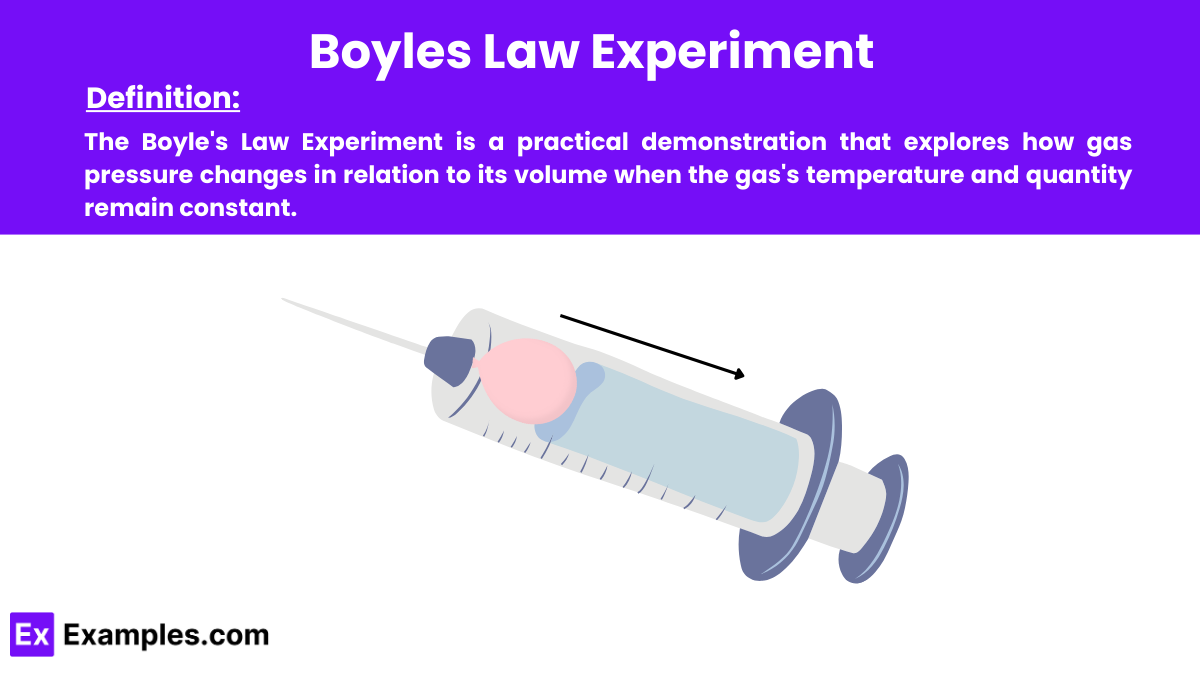
Boyle’s Law Experiment demonstrates the relationship between the pressure and volume of a gas, as stated in Boyle’s Law – the pressure of a gas is inversely proportional to its volume when temperature and the amount of gas remain constant. In other words, as the volume decreases, the pressure increases, and vice versa.
The theory behind the Boyle’s Law Experiment is rooted in the principles of gas behavior under constant temperature conditions. Boyle’s Law is one of the fundamental gas laws that describe the relationship between the pressure and volume of a gas. The law asserts that the pressure of a given amount of gas is inversely proportional to its volume, provided the temperature remains constant.
Inverse Relationship: Boyle’s Law states that as the volume of a gas decreases. Its pressure increases, and vice versa, assuming a constant temperature. This relationship is mathematically expressed as:
Molecular Theory: According to the kinetic theory of gases, gas molecules move rapidly and collide with the container walls, creating pressure. When the gas volume decreases, the gas molecules have less space to move around, causing more frequent collisions with the container walls. Which results in increased pressure.
Isothermal Process: In an isothermal process, the experiment maintains a constant temperature during volume changes. This process keeps the gas’s internal energy the same. Ensuring that the pressure-volume relationship remains inversely proportional.
Setup:
Initial Measurement:
Change Volume:
Record Pressure:
Repeat and Gather Data:
Plot Data:
Analysis:
Boyle’s Law states that at constant temperature, the pressure of a gas is inversely proportional to its volume. If one increases, the other decreases.
It helps us understand gas behavior under varying pressures and volumes, which is essential in designing equipment like syringes, pneumatic systems, and respiratory devices.
The experiment requires a constant temperature. Fluctuating temperatures introduce variables that disrupt the inverse relationship between pressure and volume.
A sealed syringe or piston-cylinder assembly, a pressure gauge or manometer, and a setup that allows for accurate volume adjustments and pressure measurements.
Use well-fitted, sealed connections and test for leaks by applying soapy water to joints and looking for bubbles when pressure is applied.
As the volume decreases, the gas molecules collide more frequently, increasing pressure. Expanding the volume reduces collisions, decreasing pressure.
A graph plotting pressure against the inverse of volume (1/V) should yield a straight line if the data aligns with Boyle’s Law.
No, Boyle’s Law specifically applies to gases because of their compressibility. Liquids and solids don’t change volume significantly under pressure.
It is used in scuba diving, automobile engines, and medical devices like syringes and ventilators, where controlling gas pressure and volume is crucial.
By maintaining constant temperature during volume changes, the experiment demonstrates an isothermal process where internal energy remains stable, proving the inverse pressure-volume relationship.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does Boyle's Law state about the relationship between pressure and volume of a gas at constant temperature?
Directly proportional
Inversely proportional
No relationship
Linearly proportional
Which of the following equations represents Boyle’s Law?
PV = nRT
P1V1 = P2V2
V/T = k
P/T = k
In a Boyle's Law experiment, if the initial pressure is doubled, what happens to the volume?
It remains the same
It is halved
It doubles
It quadruples
During a Boyle's Law experiment, the volume of a gas is reduced from 4 L to 2 L. What happens to the pressure?
It remains the same
It is halved
It doubles
It quadruples
What is kept constant in a Boyle’s Law experiment?
Volume
Pressure
Temperature
Mass
If the initial pressure of a gas is 100 kPa and the volume is 1 L, what is the final pressure if the volume changes to 0.5 L?
50 kPa
100 kPa
200 kPa
400 kPa
In a Boyle’s Law experiment, the initial pressure and volume are 300 kPa and 3 L, respectively. If the final pressure is 150 kPa, what is the final volume?
1.5 L
3 L
6 L
9 L
How would you describe the graph of pressure versus volume for a gas obeying Boyle’s Law?
Linear
Exponential
Hyperbolic
Parabolic
In Boyle’s Law, what happens to the pressure if the volume of a gas is tripled while keeping temperature constant?
It remains the same
It is tripled
It is reduced to one-third
It is doubled
Why is it important to keep the temperature constant in a Boyle’s Law experiment?
To keep the mass constant
To isolate the relationship between pressure and volume
To allow the gas to expand
To prevent changes in the number of moles
Before you leave, take our quick quiz to enhance your learning!

