What is the definition of a Torr?
A unit of length
A unit of time
A unit of pressure
A unit of temperature

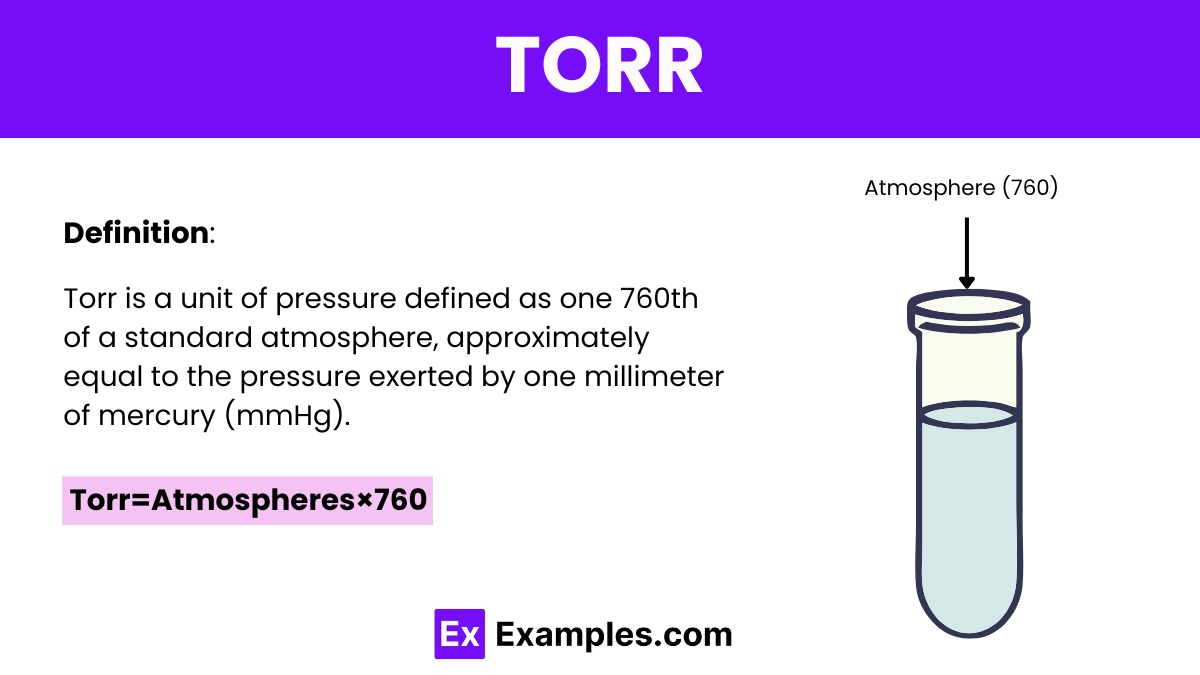
Torr is a unit of pressure based on an absolute scale, defined as 1/760 of a standard atmosphere, approximately equivalent to the pressure exerted by a millimeter of mercury (mmHg). In other units, one Torr is roughly equal to 133.322 pascals (Pa), 0.00133322 Bars, or 0.0193368 pounds per square inch (psi). It is commonly used in vacuum physics and other applications requiring fine measurements of pressure. Named after Evangelista Torricelli, the inventor of the barometer, the Torr helps bridge practical laboratory work with the theoretical calculations in physics, maintaining relevance in both scientific and industrial settings.
This formula simply multiplies the pressure in atmospheres by 760 to convert it to Torr. This is because 1 atm equals 760 Torr by definition. Thus, if you know the pressure in atmospheres, you can easily calculate the pressure in Torr using this multiplication factor.
In the food industry, vacuum packaging is a common method to extend the shelf life of products by removing air from the package, which helps to inhibit the growth of bacteria and other spoilage organisms. The effectiveness of the vacuum process is often measured in Torr to ensure that the pressure is low enough to achieve desired preservation results.
Scenario: A food processing plant needs to determine the effectiveness of their vacuum packaging machine. The machine specifications state that it should achieve a vacuum level of 50 Torr to ensure optimal food preservation.
Application: The technicians use a vacuum gauge that reads in Torr to monitor the vacuum level during the packaging process. After starting the machine, they observe that the gauge stabilizes at 50 Torr, indicating that the internal pressure of the packaging is sufficiently low to retard spoilage effectively.
In this scenario, using Torr as the measurement unit allows precise control over the packaging environment, directly influencing product quality and safety. This example demonstrates how Torr is practically applied in industry to manage and optimize processes that depend on specific pressure conditions.
Gas pressure measured in Torr provides a practical way to understand pressure systems, particularly in contexts where precise measurements of low pressure are crucial, such as in scientific laboratories or industrial processes. Here’s an explanation with a real-world example:
Scenario:
In a physics laboratory, researchers are conducting experiments in a vacuum chamber where precise control of the environment is essential. These experiments might include studying the behavior of gases at low pressures or observing chemical reactions that occur only in near-vacuum conditions.
Application:
The laboratory vacuum chamber is equipped with a pressure gauge that measures in Torr to monitor and adjust the gas pressure accurately. For a particular experiment, researchers aim to reduce the internal pressure of the chamber to 10 Torr to simulate space-like conditions.
Initial Setup: The chamber starts at atmospheric pressure, which is approximately 760 Torr.
Vacuum Pump Activation: The researchers activate the vacuum pump, and the pressure begins to drop. They monitor the gauge closely.
Reaching Target Pressure: After a period, the gauge reads 10 Torr, indicating that most of the air has been evacuated and the desired vacuum level has been achieved.
Conducting Experiments: With the chamber stabilized at 10 Torr, the researchers proceed with their experiments, able to replicate conditions that are critical for their research.
High vacuum conditions are typically measured in Torr, which is crucial for many scientific and industrial applications where extremely low pressures are required. Here’s how high vacuum is typically used, with a specific example to illustrate:
Scenario: In the production of semiconductors, maintaining a high vacuum environment is essential. This controlled environment prevents contamination and allows for the precise deposition of materials onto silicon wafers.
Application: During the process of physical vapor deposition (PVD), a high vacuum is needed to ensure that the vaporized metals can be deposited thinly and evenly without interference from air molecules.
| SI Prefix | Symbol | Multiplier to Pascal | Equivalent in Torr |
|---|---|---|---|
| Kilo- | kPa | 10³ Pa | 1 kPa = 7.50062 Torr |
| Mega- | MPa | 10⁶ Pa | 1 MPa = 7500.62 Torr |
| Giga- | GPa | 10⁹ Pa | 1 GPa = 7,500,620 Torr |
| Milli- | mPa | 10−3 Pa | 1 mPa = 0.00750062 Torr |
| Micro- | µPa | 10⁻³ Pa | 1 µPa = 0.00000750062 Torr |
| Nano- | nPa | 10⁻⁹ Pa | 1 nPa = 0.00000000750062 Torr |
| Pico- | pPa | 10⁻¹² Pa | 1 pPa = 0.00000000000750062 Torr |
| Femto- | fPa | 10⁻¹⁵ Pa | 1 fPa = 0.00000000000000750062 Torr |
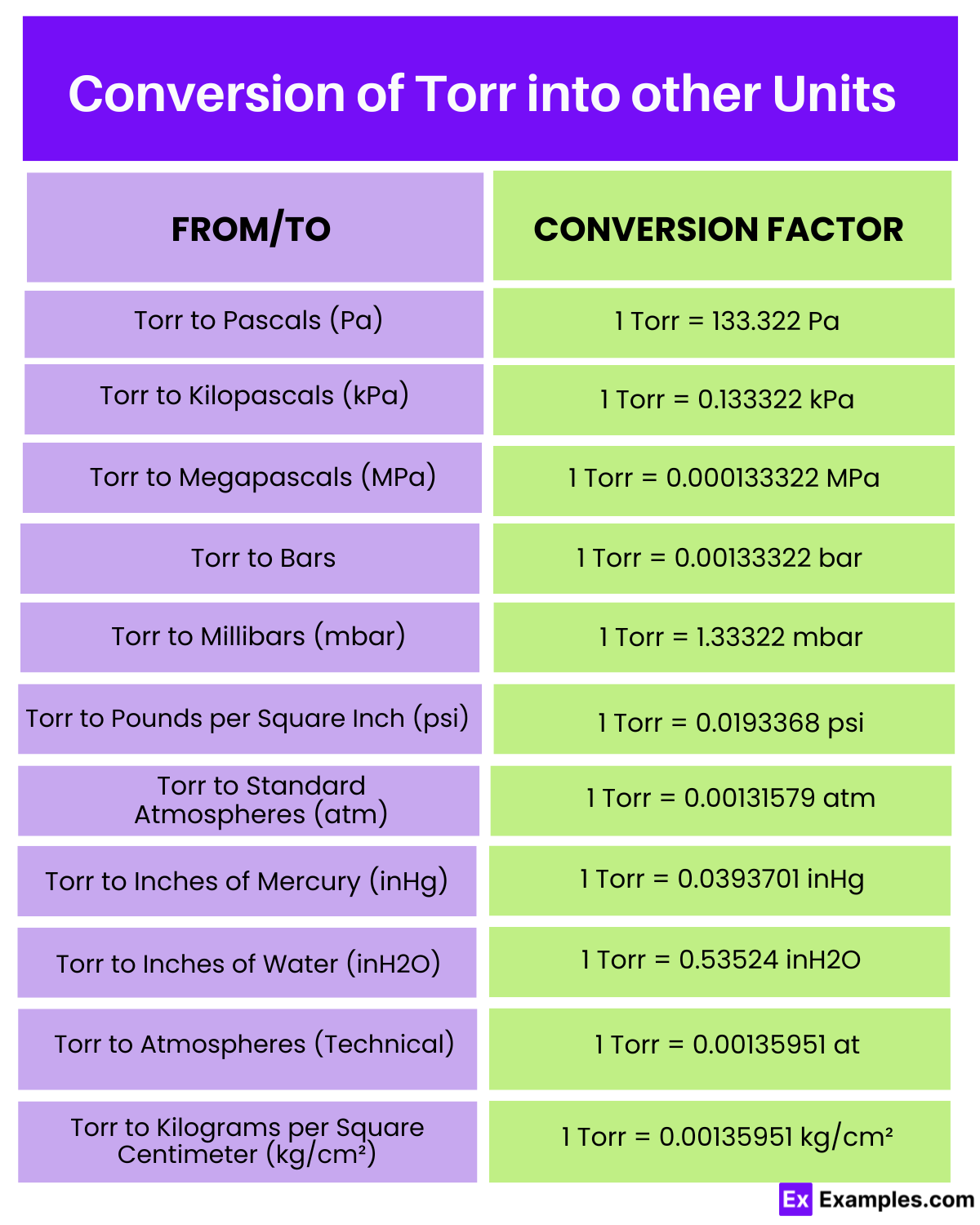
| To Unit | Conversion Factor | Conversion from 10 Torr |
|---|---|---|
| Torr to Pascals (Pa) | 1 Torr = 133.322 Pa | 10 Torr = 1,333.22 Pa |
| Torr to Kilopascals (kPa) | 1 Torr = 0.133322 kPa | 10 Torr = 1.33322 kPa |
| Torr to Megapascals (MPa) | 1 Torr = 0.000133322 MPa | 10 Torr = 0.00133322 MPa |
| Torr to Bars | 1 Torr = 0.00133322 bar | 10 Torr = 0.0133322 bar |
| Torr to Millibars (mbar) | 1 Torr = 1.33322 mbar | 10 Torr = 13.3322 mbar |
| Torr to Pounds per Square Inch (psi) | 1 Torr = 0.0193368 psi | 10 Torr = 0.193368 psi |
| Torr to Standard Atmospheres (atm) | 1 Torr = 0.00131579 atm | 10 Torr = 0.0131579 atm |
| Torr to Inches of Mercury (inHg) | 1 Torr = 0.0393701 inHg | 10 Torr = 0.393701 inHg |
| Torr to Inches of Water (inH2O) | 1 Torr = 0.53524 inH2O | 10 Torr = 5.3524 inH2O |
| Torr to Atmospheres (Technical) | 1 Torr = 0.00135951 at | 10 Torr = 0.0135951 at |
| Torr to Kilograms per Square Centimeter (kg/cm²) | 1 Torr = 0.00135951 kg/cm² | 10 Torr = 0.0135951 kg/cm² |
This conversion table demonstrates how 10 Torr translates into various pressure units, from Pascals to Kilograms per Square Centimeter, facilitating accurate and universal pressure measurements across diverse scientific and technical applications. Each unit caters to specific measurement needs, ensuring precision and consistency in data across fields.
Torr converts to pascals, the SI unit for pressure, which is widely used in most scientific and engineering calculations involving force applied over an area.
These are larger units of pascals, scaling up for convenience in handling larger or industrial-scale pressures. They are used for applications requiring a broader scale, such as structural engineering and materials science.
These are larger units of pascals, scaling up for convenience in handling larger or industrial-scale pressures. They are used for applications requiring a broader scale, such as structural engineering and materials science.
Common in meteorology, the bar is nearly equivalent to atmospheric pressure at sea level. Millibars offer a finer scale, crucial for detailed atmospheric pressure measurements that drive weather prediction models.
Common in meteorology, the bar is nearly equivalent to atmospheric pressure at sea level. Millibars offer a finer scale, crucial for detailed atmospheric pressure measurements that drive weather prediction models.
This unit is predominant in the United States for industrial and consumer applications, including automotive tire pressures and hydraulic systems.
The atm unit is closely aligned with the average atmospheric pressure at sea level, making it practical for studying and modeling atmospheric conditions in environmental sciences.
Often used in aviation and meteorology, this unit relates to historical barometric pressure measurements made using mercury columns.
Typically used in applications involving low pressures, such as in HVAC systems and medical ventilators, where precision in slight pressure variations is crucial.
A variant of the standard atmosphere, this unit is used primarily in engineering to measure pressures in hydraulic and pneumatic systems.
This unit is common in some industrial applications outside of the U.S., particularly in hydraulics and materials testing, where it relates pressure directly to weight force per unit area.
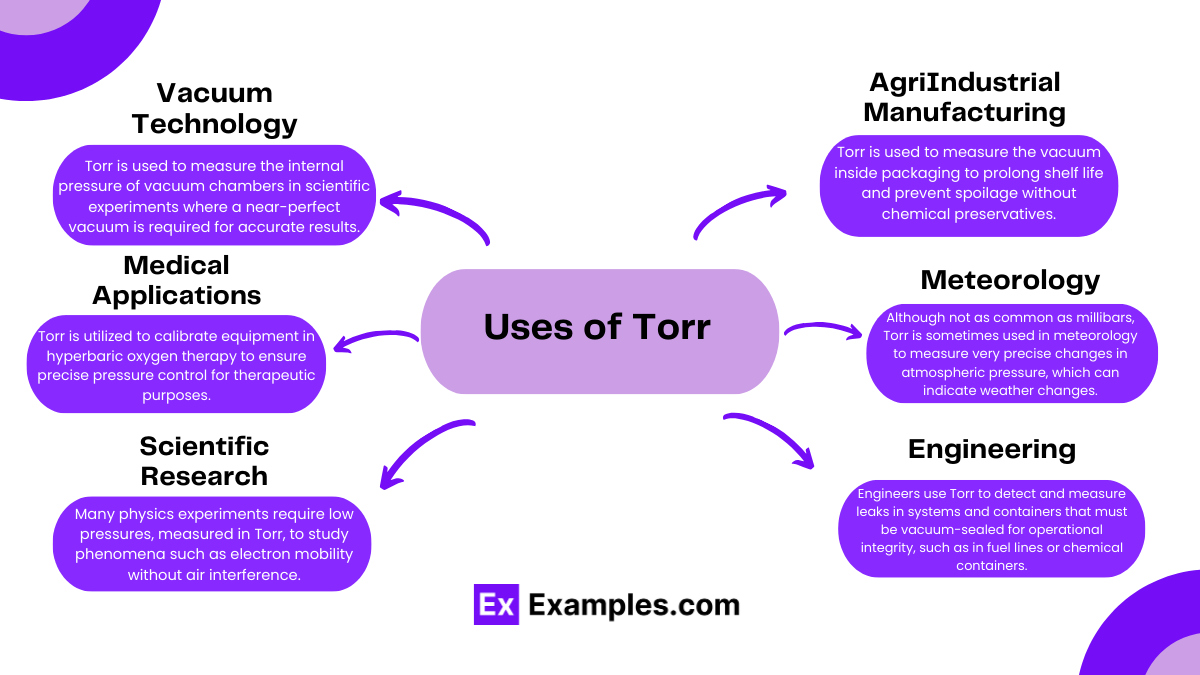
Leak Detection: Engineers use Torr to detect and measure leaks in systems and containers that must be vacuum-sealed for operational integrity, such as in fuel lines or chemical containers.
Torr is extremely useful in vacuum applications because it allows for precise measurement of very low pressures, which are common in scientific laboratories and industrial processes that require a controlled vacuum environment.
Unlike psi, which measures pressure relative to the atmosphere, or pascal, which is the SI unit of pressure, Torr is specifically scaled to the millimeter of mercury measurement. This makes it particularly suitable for applications like vacuum measurements where fine precision is needed.
Torr is widely used in fields such as meteorology for high-altitude pressure measurement, aerospace for testing space simulation chambers, and in medical settings for calibrating suction devices and anesthesia equipment.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the definition of a Torr?
A unit of length
A unit of time
A unit of pressure
A unit of temperature
How many Torr are there in 1 atmosphere (atm)?
1
100
760
1013
If the pressure of a gas is 380 Torr, what is the pressure in atmospheres?
0.25 atm
0.50 atm
1 atm
2 atm
Which of the following pressures is equivalent to 1520 Torr?
1 atm
1.5 atm
2 atm
2.5 atm
What is the pressure in Torr if the pressure is 2 atmospheres?
380 Torr
760 Torr
1140 Torr
1520 Torr
Which instrument is used to measure pressure in Torr?
Thermometer
Barometer
Hygrometer
Voltmeter
If a vacuum pump reduces the pressure in a chamber to 50 Torr, what is the pressure in mmHg?
50 mmHg
100 mmHg
200 mmHg
500 mmHg
A gas has a pressure of 1520 Torr. What is this pressure in kilopascals (kPa)? (1 atm = 101.325 kPa)
101.325 kPa
152.0 kPa
202.65 kPa
304.95 kPa
How does the pressure in Torr change if the volume of a gas is halved at constant temperature (Boyle’s Law)?
It remains the same
It is halved
It is doubled
It is quadrupled
If the pressure of a gas is measured as 500 Torr, what is the pressure in mmHg?
500 mmHg
250 mmHg
750 mmHg
1000 mmHg
Before you leave, take our quick quiz to enhance your learning!

