What is the atomic number of darmstadtium?
108
109
110
111
Embark on a captivating journey into the world of chemistry with our comprehensive guide to Darmstadtium, a synthetic element that has intrigued scientists and researchers alike. Delve into the nuances of its discovery, properties, and the groundbreaking uses and compounds it has inspired. This guide is designed to enlighten both novices and seasoned chemists, offering insightful examples that highlight the significance of Darmstadtium in modern scientific endeavors. Explore the fascinating realm of this elusive element, and discover its potential to revolutionize various fields.
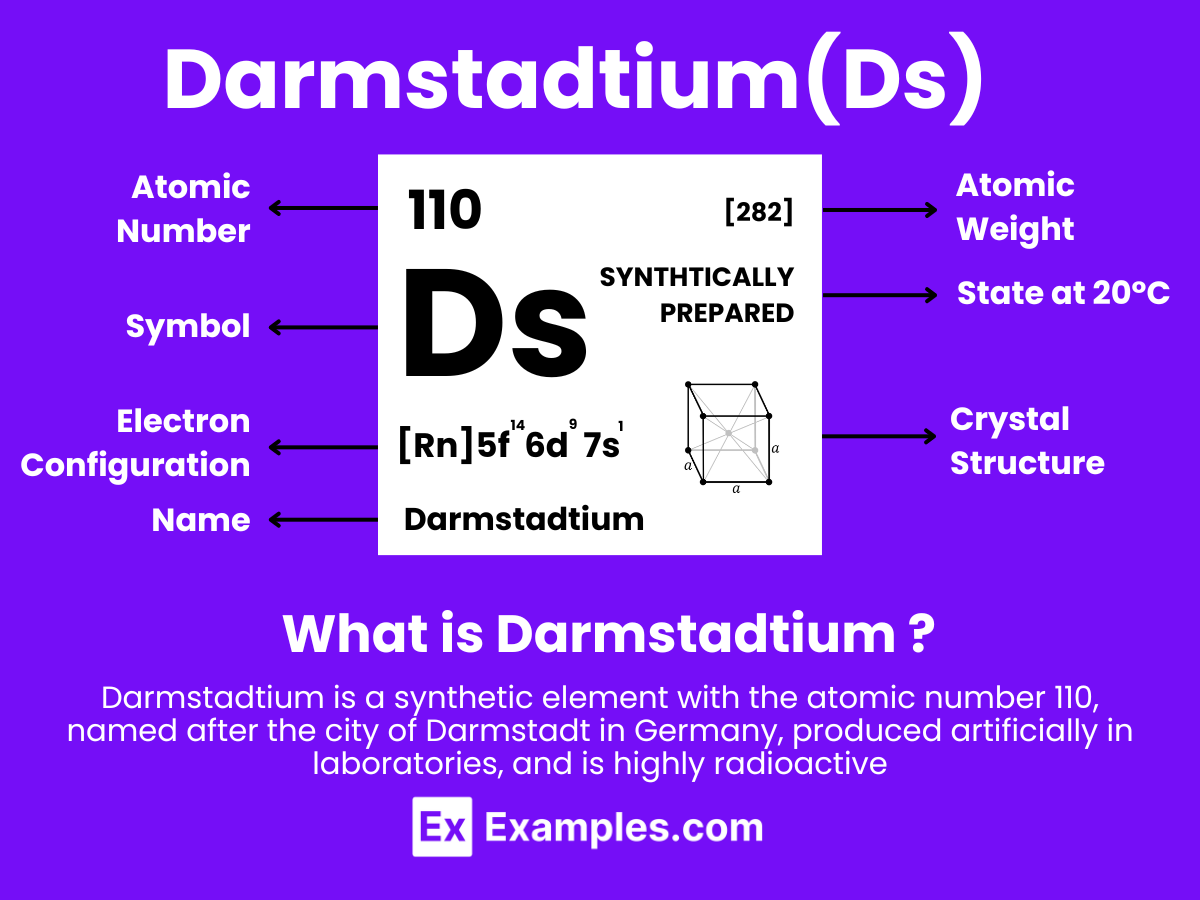
Whats is Darmstadtium ?
Darmstadtium is a synthetic chemical element with the symbol Ds and atomic number 110. It is a highly radioactive metal, of which only a few atoms have been created, and it does not occur naturally. Darmstadtium was first synthesized in 1994 by a team of German scientists at the GSI Helmholtz Centre for Heavy Ion Research near Darmstadt, Germany, which is how it got its name. Because only small amounts have been produced and it has a very short half-life, darmstadtium’s chemical and physical properties are not well understood. Its position in the periodic table suggests it would have properties similar to those of other group 10 elements (nickel, palladium, and platinum).
Formula: Ds
Composition: A single darmstadtium atom.
Bond Type: As a highly radioactive and short-lived element, darmstadtium’s bonding characteristics are not well-studied, but it is expected to form covalent bonds if it were to form compounds.
Molecular Structure: Darmstadtium is a synthetic element and has no stable or naturally occurring isotopes, making its physical properties difficult to ascertain. However, as a member of the transition metals, it might share similarities with other elements in its group.
Electron Configuration: 110 electrons, with a theoretical configuration of [Rn] 5f¹⁴ 6d⁸ 7s², assuming it follows the expected trends within its group.
Significance: Darmstadtium’s primary use is in scientific research, particularly in the study of nuclear physics and the synthesis of new elements. Its production and decay properties offer insights into the behavior of superheavy atomic nuclei.
Role in Chemistry: Darmstadtium’s role is mainly experimental due to its extreme rarity and instability. It contributes to our understanding of the periodic table’s limits and the formation of elements beyond uranium.
Darmstadtium, unlike hydrogen, is a synthetic metal with assumed distinctive characteristics, reflecting its position as a superheavy element in the periodic table. Due to its highly unstable nature and extremely short half-life, the physical properties of darmstadtium, including its melting and boiling points, are largely speculative and not directly observable. However, theoretical considerations can provide insight into its behavior at the atomic and molecular levels.
Atomic Level: Each darmstadtium atom (Ds) contains 110 protons in its nucleus and is theorized to have 110 electrons orbiting around it. The electron configuration of darmstadtium is predicted to be [Rn] 5f¹⁴ 6d⁸ 7s², indicating it has ten electrons in its outermost shells that could be available for bonding, in theory.
Molecular Formation: As a superheavy element, darmstadtium does not form molecules in the same way lighter elements like hydrogen do. Given its extremely short half-life and the conditions under which it exists, it’s challenging to predict its state with certainty. However, if darmstadtium atoms could form a bulk phase, they might arrange in a crystalline or possibly more complex lattice structure when solid, involving metallic bonding characteristics similar to those observed in other transition metals. Such bonding would involve the delocalization of electrons across many darmstadtium atoms, distinct from the covalent bonding in hydrogen molecules.
Given the speculative nature of darmstadtium’s properties, it’s hypothesized that, like other transition metals, it would have a high melting point and high boiling point, indicative of strong bonds within its lattice. However, due to its rapid decay, darmstadtium does not exist long enough to observe such states or to form a metallic lattice under normal laboratory conditions. It is synthesized in particle accelerators and detected almost instantaneously as it decays into other elements.
Comparative Analysis: Unlike hydrogen, which is a simple, naturally occurring diatomic gas at room temperature, darmstadtium’s existence is fleeting and artificial, created in highly controlled laboratory conditions. It does not exist naturally in any state due to its rapid decay, making it impossible to observe or measure properties like melting or boiling points directly. Theoretical predictions suggest it would behave more similarly to other group 10 metals, with potentially complex and dense atomic arrangements, if it could be stabilized long enough to form a bulk material.
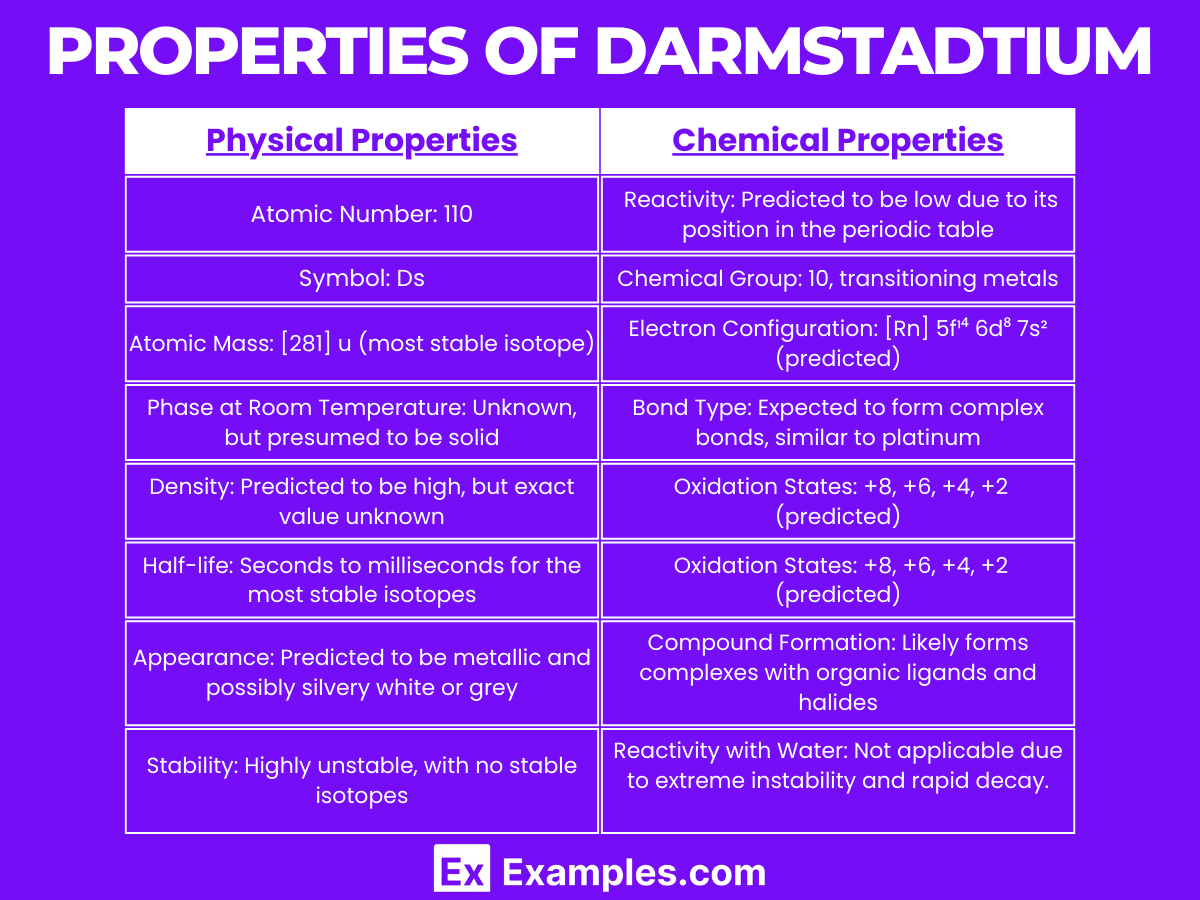
| Physical Property | Value/Description |
|---|---|
| Atomic Number | 110 |
| Symbol | Ds |
| Atomic Mass | [281] u (most stable isotope) |
| Phase at Room Temperature | Presumed solid (theoretical) |
| Density (predicted) | High, exact value unknown |
| Half-life (most stable isotope) | Seconds to milliseconds |
| Appearance (predicted) | Metallic, possibly silvery white or grey |
| Stability | Highly unstable, no stable isotopes |
he chemical properties of Darmstadtium (Ds), a synthetic element with the atomic number 110, are largely speculative due to its extremely short half-life and the very small amounts in which it is produced. As a member of group 10 in the periodic table, it is expected to share some chemical properties with its lighter homologues, such as nickel (Ni), palladium (Pd), and platinum (Pt). Here is a detailed exploration of the anticipated chemical properties of Darmstadtium:
Darmstadtium’s reactivity is predicted to be similar to that of other group 10 elements, potentially forming complexes with organic ligands and reacting with halogens. However, the exact nature of its reactivity remains theoretical.
The predicted electron configuration for the most stable isotope of Darmstadtium, Ds-281, is [Rn] 5f¹⁴ 6d⁸ 7s². This configuration suggests that Darmstadtium could exhibit a range of oxidation states, with +8, +6, +4, and +2 being the most likely, similar to platinum.
Based on its position in the periodic table and the behavior of its group 10 counterparts, Darmstadtium is expected to exhibit multiple oxidation states. Its most stable and common oxidation state is predicted to be +6, though the element’s highly unstable nature makes this difficult to verify experimentally.
Darmstadtium is expected to form bonds that are covalent in nature due to its anticipated high electronegativity and ionization energy. The element may form complex compounds with a variety of ligands, similarly to how platinum forms square planar complexes.
While no compounds of Darmstadtium have been synthesized or observed due to its short half-life, theoretical studies suggest it could form compounds such as DsO₂ or DsCl₆, following the trends observed in the chemistry of platinum and other group 10 elements.
Due to the extremely limited amount of time that Darmstadtium exists before decaying, its behavior with water, air, and other common substances has not been observed. It is hypothesized that, like platinum, Darmstadtium would be relatively inert, but this remains speculative.
A hypothetical reaction, based on the predicted chemistry of Darmstadtium, could be its reaction with fluorine to form a hexafluoride, similar to platinum:Ds+3F₂→DsF₆
This equation is speculative and serves to illustrate the type of chemical behavior that might be expected from Darmstadtium if it were possible to observe its reactions.
In summary, while the chemical properties of Darmstadtium can be predicted based on its position in the periodic table and the properties of similar elements, the extremely short half-life of its isotopes prevents the practical observation and confirmation of these properties.
| Material Property | Darmstadtium (Theoretical Values) |
|---|---|
| Atomic Number | 110 |
| Symbol | Ds |
| Atomic Mass | [281] u (most stable isotope) |
| State at Room Temperature | Presumed Solid |
| Density (Predicted) | High, but specific value unknown |
| Melting Point | Unknown; predicted to be high |
| Boiling Point | Unknown; predicted to be high |
| Color | Presumed to be metallic and silvery |
| Electromagnetic Property | Value/Description (Theoretical) |
|---|---|
| Electrical Conductivity | Predicted to be high, similar to other transition metals |
| Magnetic Susceptibility | Theoretical; likely paramagnetic or diamagnetic depending on its most stable oxidation state |
| Superconductivity | Unknown; if present, would likely occur at extremely low temperatures |
| Magnetoresistance | Not available; speculative comparison could be made with platinum group metals |
| Work Function | Theoretical; expected to be comparable to platinum and palladium |
| Electron Affinity | Predicted based on group trends; exact value unknown |
| Ionization Energy | High; consistent with heavy transition metals but exact value is speculative |
| Reflectivity | Likely high, indicative of a shiny, metallic appearance |
| Nuclear Property | Value/Description |
|---|---|
| Atomic Number | 110 |
| Mass Number (most stable isotope) | 281 (for Ds-281, the most stable isotope known) |
| Half-life of Most Stable Isotope | Approximately 10 seconds (for Ds-281) |
| Type of Decay | Primarily alpha decay, some isotopes may undergo spontaneous fission |
| Decay Products | Various, including isotopes of hassium (Hs) |
| Neutron Cross Section | Not measured due to short half-life |
| Neutron Number (for Ds-281) | 171 |
| Isotopes Discovered | Several, with mass numbers ranging from 267 to 282 |
Darmstadtium hexafluoride (DsF₆): A highly reactive, colorless gas similar to platinum hexafluoride, expected to be a strong oxidizing agent.
Equation: Ds + 3F₂ → DsF₆
Darmstadtium chloride (DsCl₂): A dimeric compound in solid form, analogous to platinum(II) chloride, potentially used in catalysis research.
Equation: Ds + Cl₂ → DsCl₂
Darmstadtium oxide (DsO₂): A solid compound, expected to have a crystalline structure similar to the oxides of platinum, showing moderate reactivity.
Equation: Ds + O₂ → DsO₂
Darmstadtium hexachloride (DsCl₆): Theoretically, a highly reactive compound that would be challenging to stabilize, reflecting the behavior of platinum hexachloride.
Equation: Ds + 3Cl₂ → DsCl₆
Darmstadtium tetrahydride (DsH₄): A gaseous compound, speculated to be similar to nickel tetrahydride, with significant interest for bonding studies.
Equation: Ds + 2H₂ → DsH₄
Darmstadtium carbonyl (Ds(CO)₄): A volatile compound expected to exhibit strong metal-to-ligand back-donation, similar to its lighter homologs in organometallic chemistry.
Equation: Ds + 4CO → Ds(CO)₄
Here is a table summarizing some of the known isotopes of darmstadtium (Ds), including their mass numbers, half-lives, and decay modes. This table represents a snapshot of our current understanding and may expand as new isotopes are discovered and characterized.
| Isotope | Mass Number | Half-Life | Decay Mode(s) |
|---|---|---|---|
| Ds-267 | 267 | 10 microseconds | Alpha decay to ²⁶³Hs |
| Ds-268 | 268 | 100 microseconds | Alpha decay to ²⁶⁴Hs |
| Ds-269 | 269 | 180 microseconds | Alpha decay to ²⁶⁵Hs |
| Ds-270 | 270 | 100 microseconds | Alpha decay to ²⁶⁶Hs |
| Ds-271 | 271 | 1.63 milliseconds | Alpha decay to ²⁶⁷Hs |
| Ds-273 | 273 | 170 milliseconds | Alpha decay to ²⁶⁹Hs |
| Ds-279 | 279 | 0.18 seconds | Alpha decay to ²⁷⁵Hs |
| Ds-281 | 281 | 11 seconds | Alpha decay to ²⁷⁷Hs |
In conclusion, darmstadtium represents a pinnacle of human ingenuity in nuclear science, marking significant advances in our understanding of the periodic table’s limits. Although its fleeting existence precludes practical applications, the synthesis and study of darmstadtium’s isotopes enhance our knowledge of nuclear stability and the potential for discovering new elements within the theoretical “island of stability.
Text prompt
Add Tone
Isotopes of Darmstadtium
Uses of Darmstadtium
What is the atomic number of darmstadtium?
108
109
110
111
In which year was darmstadtium first synthesized?
1990
1994
2000
2004
What is the symbol for darmstadtium?
Ds
Dm
Da
Dt
Darmstadtium belongs to which group in the periodic table?
Group 8
Group 9
Group 10
Group 11
Which of the following is the most stable isotope of darmstadtium?
Ds-267
Ds-271
Ds-277
Ds-281
What is the primary method used to synthesize darmstadtium?
Chemical reactions
Nuclear fusion
Electrolysis
Radioactive decay
Darmstadtium is expected to exhibit similar chemical properties to which element?
Copper
Silver
Gold
Platinum
Darmstadtium was first synthesized at which facility?
Lawrence Berkeley National Laboratory
GSI Helmholtz Centre for Heavy Ion Research
CERN
Fermilab
What is the half-life of the most stable isotope of darmstadtium, Ds-281?
1 second
11 seconds
20 seconds
30 seconds
Which type of decay is darmstadtium most likely to undergo?
Alpha decay
Beta decay
Gamma decay
Positron emission
Before you leave, take our quick quiz to enhance your learning!

