What is the atomic number of gadolinium?
62
63
64
65
Gadolinium, a lanthanide series element, is renowned for its exceptional properties and versatile applications, making it a subject of interest across various scientific and technological fields. This comprehensive guide delves into the world of Gadolinium, exploring its definition, significance, and the myriad of uses and compounds associated with it. With practical examples, we aim to enrich your understanding of this fascinating element, highlighting its role in enhancing technological advancements and medical innovations. Discover the unique attributes and transformative potential of Gadolinium in our detailed exploration.
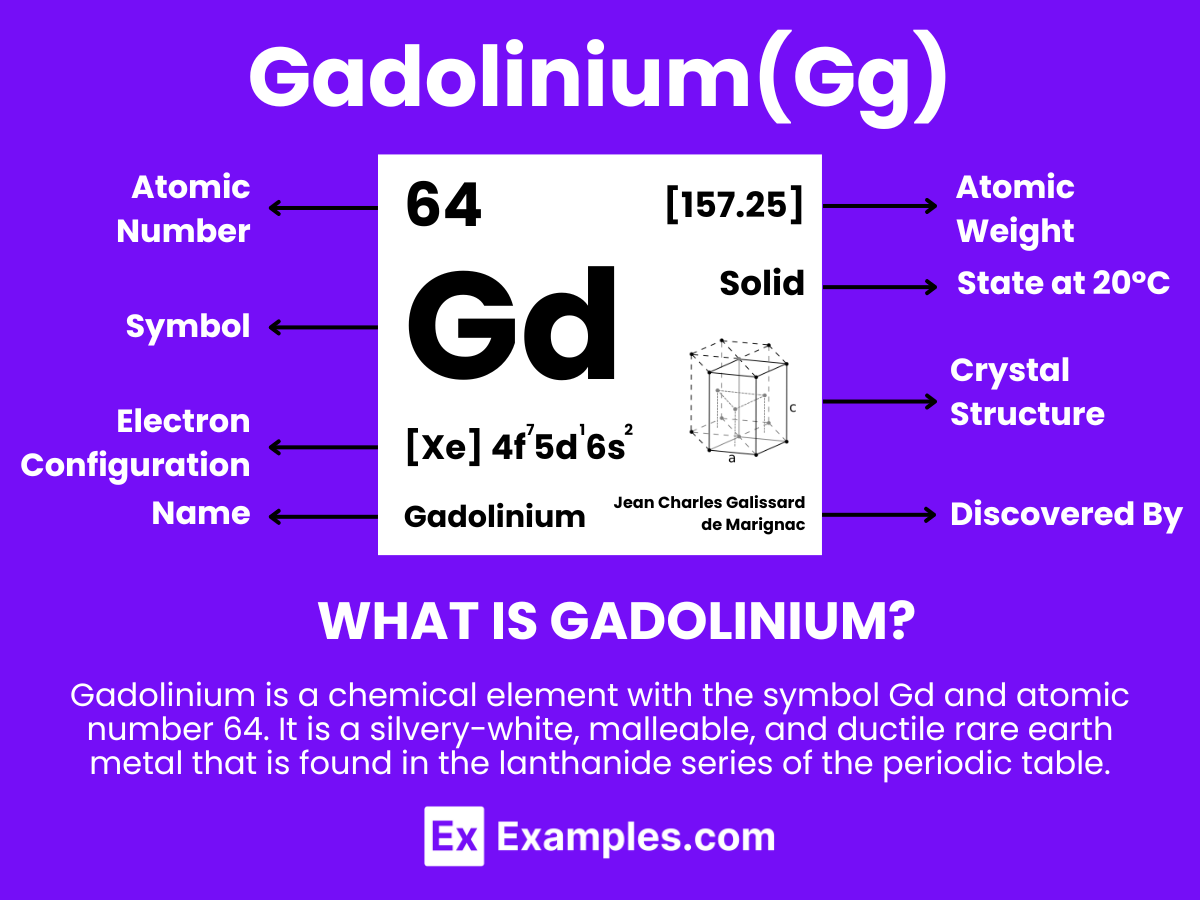
Gadolinium is a chemical element with the symbol Gd and atomic number 64. It is a silvery-white, malleable, and ductile rare earth metal that is found in the lanthanide series of the periodic table. Gadolinium possesses unique chemical and physical properties, including a high magnetic susceptibility, making it especially useful in various applications within the fields of electronics, manufacturing, and medicine.One of the most notable properties of gadolinium is its use in magnetic resonance imaging (MRI) as a contrast agent, enhancing the quality of the images obtained. This is due to its ability to improve the visibility of internal body structures by altering the magnetic properties of water molecules in the human body under the influence of an MRI’s magnetic field.
| Property | Value |
|---|---|
| Appearance | Silvery white, metallic |
| Atomic Mass | 157.25 u |
| Density | 7.90 g/cm³ at 20°C |
| Melting Point | 1313 °C |
| Boiling Point | 3273 °C |
| Magnetic Ordering | Ferromagnetic below 20°C, Paramagnetic above |
| Specific Heat Capacity | 240 J/(kg·K) |
| Thermal Conductivity | 10.6 W/(m·K) |
| Electrical Resistivity | 1.310 µΩ·m (at 25 °C) |
Gadolinium exhibits a range of chemical properties that underscore its reactivity and its ability to form various compounds. Below are detailed insights into its chemical nature, including relevant reactions and equations:
| Property | Value |
|---|---|
| Melting Point | 1313 °C |
| Boiling Point | 3273 °C |
| Specific Heat Capacity | 240 J/(kg·K) |
| Thermal Conductivity | 10.6 W/(m·K) |
| Thermal Expansion | 9.4 µm/(m·K) at 25 °C |
| Heat of Fusion | 10.05 kJ/mol |
| Heat of Vaporization | 301.3 kJ/mol |
| Entropy of Fusion | 14.5 J/(mol·K) at the melting point |
| Property | Value |
|---|---|
| Density | 7.90 g/cm³ at 20 °C |
| Mohs Hardness | ~5.1 |
| Young’s Modulus | 54.8 GPa |
| Shear Modulus | 21.8 GPa |
| Bulk Modulus | 37.9 GPa |
| Poisson Ratio | 0.259 |
| Brinell Hardness | 570 – 740 HB |
| Vickers Hardness | Similar to Brinell Hardness |
| Property | Value |
|---|---|
| Magnetic Ordering | Ferromagnetic below 20°C, Paramagnetic above |
| Curie Temperature | ~20 °C (293 K) |
| Magnetic Susceptibility | High at low temperatures |
| Electrical Resistivity | 1.310 µΩ·m at 25 °C |
| Superconducting Point | Below 1.083 K (under high pressure) |
| Property | Value |
|---|---|
| Natural Isotopes | ¹⁵²Gd, ¹⁵⁴Gd, ¹⁵⁵Gd, ¹⁵⁶Gd, ¹⁵⁷Gd, ¹⁵⁸Gd, ¹⁶⁰Gd |
| Most Stable Isotopes | ¹⁶⁰Gd (half-life: >1.3×10²¹ years) |
| Neutron Cross Section | 49,000 barns for ¹⁵⁷Gd (thermal neutrons) |
| Neutron Capture Products | Used in nuclear reactors as a neutron absorber |
| Isotopic Abundance | Varies by isotope |
1.Gadolinium Oxide (Gd₂O₃)
2.Gadolinium Chloride (GdCl₃)
3.Gadolinium Fluoride (GdF₃)
4.Gadolinium Nitrate (Gd(NO₃)₃)
5.Gadolinium Sulfide (Gd₂S₃)
6.Gadolinium Bromide (GdBr₃)
| Isotope | Mass Number | Half-Life | Mode of Decay | Application/Significance |
|---|---|---|---|---|
| ¹⁴⁸Gd | 148 | >75 years | α-decay | Scientific research |
| ¹⁵⁰Gd | 150 | >1.79×10⁶ years | α-decay | Scientific research |
| ¹⁵²Gd | 152 | Stable | – | Natural abundance |
| ¹⁵³Gd | 153 | Stable | – | Natural abundance, MRI contrast agent |
| ¹⁵⁴Gd | 154 | Stable | – | Natural abundance |
| ¹⁵⁵Gd | 155 | Stable | – | Neutron capture therapy, nuclear reactors |
| ¹⁵⁶Gd | 156 | Stable | – | Natural abundance |
| ¹⁵⁷Gd | 157 | Stable | – | Neutron capture therapy, MRI contrast agent |
| ¹⁵⁸Gd | 158 | Stable | – | Natural abundance |
| ¹⁶⁰Gd | 160 | Stable | – | Natural abundance |
The production of Gadolinium, a rare earth element with significant applications in various industries, involves a series of complex processes. These steps ensure the extraction, separation, and purification of Gadolinium from its ores, primarily from minerals such as monazite and bastnäsite, which contain mixed rare earth elements. Here’s an overview of the production process:
Gadolinium’s unique set of properties, from its use in medical imaging to its role in nuclear reactors and electronic components, underscores its indispensable role across various industries. This table of gadolinium highlights not only its versatility and utility but also its significance in advancing technology and improving human health, marking it as a critical element in modern science and engineering.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of gadolinium?
62
63
64
65
What is the chemical symbol for gadolinium?
Ga
Gd
Gm
Gn
Which of the following is a common use of gadolinium?
Jewelry making
MRI contrast agents
Fertilizers
Glass production
What is the melting point of gadolinium?
1313°C
1423°C
1585°C
1625°C
In what form is gadolinium typically found in nature?
As a free metal
In sulfide ores
In oxide minerals
In chloride salts
Which element is gadolinium most similar to in terms of chemical properties?
Iron
Calcium
Samarium
Zinc
Gadolinium is known for its high absorption cross-section for which type of radiation?
Alpha radiation
Beta radiation
Gamma radiation
Neutron radiation
Gadolinium is ferromagnetic at what temperature?
Below 0°C
Above 20°C
Below 20°C
Below 20 K
Which property of gadolinium makes it useful in cryocoolers?
High density
High melting point
Large specific heat capacity
Low thermal conductivity
Gadolinium is often alloyed with other metals to improve:
Electrical conductivity
Magnetic properties
Corrosion resistance
Hardness
Before you leave, take our quick quiz to enhance your learning!

