What is the primary use of gold in modern industries?
Electronics
Pharmaceuticals
Food additives
Fuel additives
An enlightening expedition into the world of Gold, a symbol of wealth and beauty that transcends time and culture. This comprehensive guide illuminates gold’s fundamental aspects, from its atomic structure and historical significance to its diverse applications in jewelry, electronics, and medicine. Delve into the realm of gold compounds, where chemistry meets alchemy, revealing the element’s versatility and enduring allure. With vivid examples, this guide unravels the mysteries of gold, offering insights into its enduring legacy and its pivotal role in both ancient and modern technology. Discover the golden thread that weaves through the fabric of human civilization, marking milestones of innovation and expressions of opulence.
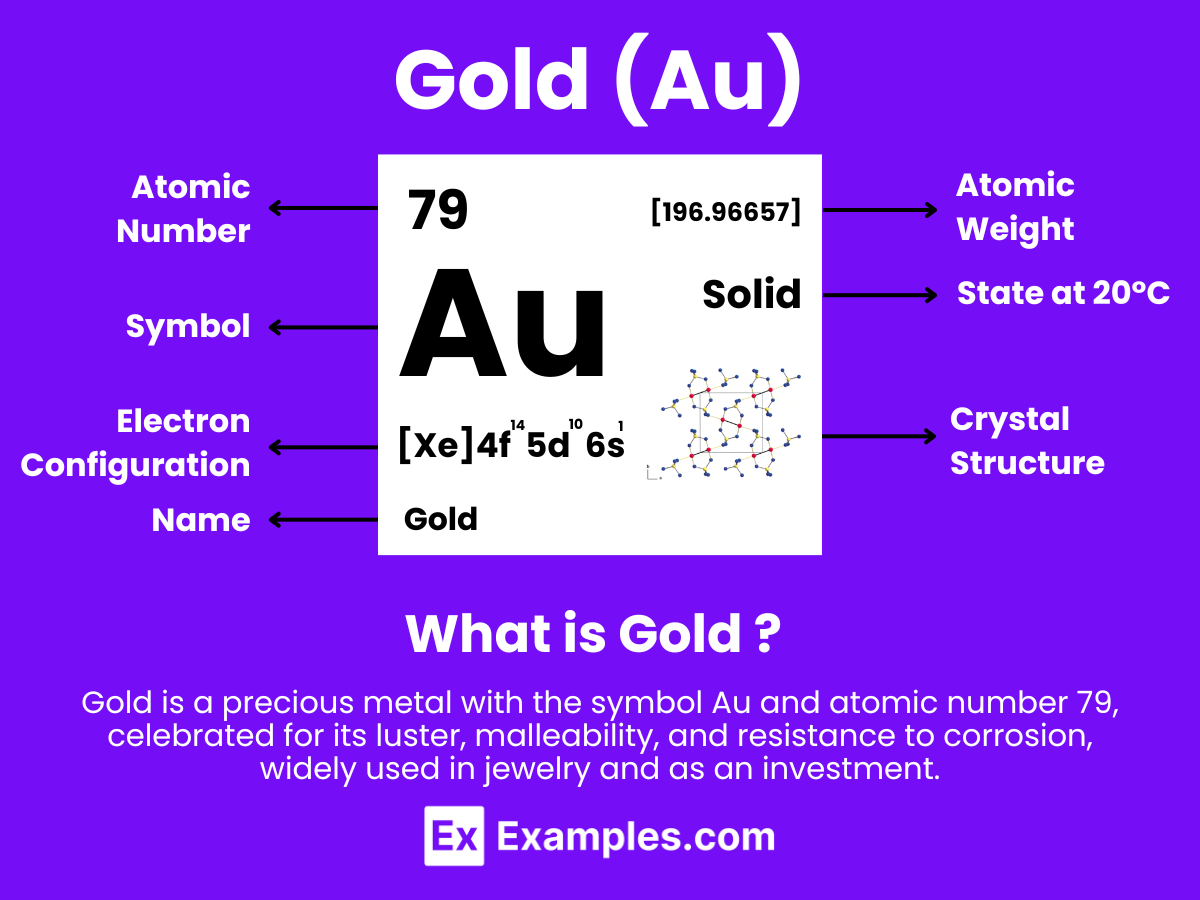
Gold is a chemical element with the symbol Au (from Latin: aurum) and atomic number 79, making it one of the higher atomic number elements that occur naturally. It is a bright, slightly reddish yellow, dense, soft, malleable, and ductile metal. Gold is a transition metal and a group 11 element. It is one of the least reactive chemical elements and is solid under standard conditions.
Gold has been used by humans for various purposes for thousands of years. Here are some key aspects and uses of gold
Gold, in contrast to hydrogen, is a metallic element with well-established characteristics that highlight its stability and versatility, particularly in solid form. The behavior of gold at the atomic and molecular levels significantly differs from that of hydrogen, due to its position as a transition metal in the periodic table and its distinct metallic characteristics.
Atomic Level: Each gold atom (Au) contains 79 protons in its nucleus and is expected to have 79 electrons orbiting around it. The electron configuration of gold is [Xe] 4f¹⁴ 5d¹⁰ 6s¹, indicating a relatively stable electron configuration that contributes to its low reactivity. Gold’s ability to exist in the +1 and +3 oxidation states, similar to other transition metals, underlines its chemical versatility and potential for forming various compounds under standard conditions.
Molecular Formation: Unlike hydrogen, which readily forms diatomic molecules (H₂) through covalent bonding, gold does not form molecules in a similar manner due to its metallic nature. In bulk form, gold atoms are arranged in a face-centered cubic lattice structure. This structure is characterized by metallic bonding, where valence electrons are free to move throughout the entire metal lattice, enabling excellent electrical conductivity and malleability. Gold’s metallic bonds differ fundamentally from the discrete electron sharing seen in hydrogen’s covalent bonds, contributing to gold’s distinct physical properties such as its lustrous appearance and ductility. Due to gold’s chemical stability and resistance to corrosion, any metallic form it takes is durable and can persist indefinitely under standard conditions, unlike the ephemeral and highly radioactive nature of superheavy elements like bohrium
| Property | Value |
|---|---|
| Appearance | Bright, slightly reddish yellow, metallic luster |
| Atomic Number | 79 |
| Atomic Mass | 196.966570(4) u |
| Melting Point | 1,064 °C (1,947 °F; 1,337 K) |
| Boiling Point | 2,970 °C (5,378 °F; 3,243 K) |
| Density (at 20 °C) | 19.32 g/cm³ |
| State at 20 °C | Solid |
| Heat of Fusion | 12.55 kJ/mol |
| Heat of Vaporization | 342 kJ/mol |
| Thermal Conductivity | 318 W/(m·K) |
| Electrical Conductivity | 45.2 MS/m |
| Malleability | Extremely malleable, can be beaten into thin sheets |
| Ductility | High, can be drawn into very thin wires |
Gold is one of the least reactive chemical elements, showing resistance to corrosion and oxidation in most environments. This inertness is one of gold’s most significant characteristics, making it highly valuable for various applications, especially in jewelry and electronics. Here are some detailed chemical properties:
| Property | Value |
|---|---|
| Melting Point | 1,064 °C (1,947 °F; 1,337 K) |
| Boiling Point | 2,970 °C (5,378 °F; 3,243 K) |
| Heat of Fusion | 12.55 kJ/mol |
| Heat of Vaporization | 342 kJ/mol |
| Specific Heat Capacity | 25.418 J/(mol·K) at 25 °C |
| Thermal Conductivity | 318 W/(m·K) |
| Thermal Expansion | 14.2 µm/(m·K) at 25 °C |
| Property | Value |
|---|---|
| Density | 19.32 g/cm³ at 20 °C |
| Mohs Hardness | Approx. 2.5 |
| Young’s Modulus | 79 GPa |
| Tensile Strength | 120 MPa |
| Malleability | Extremely high, can be flattened into sheets thinner than a micron |
| Ductility | High, can be drawn into thin wires without breaking |
| Electrical Resistivity | 22.14 nΩ·m at 20 °C |
| Electrical Conductivity | 45.2 MS/m |
| Reflectivity | Approx. 83% for infrared light |
| Electromagnetic Property | Description |
|---|---|
| Electrical Conductivity | High; gold is an excellent conductor of electricity due to its delocalized electrons. |
| Thermal Conductivity | Very high; among the highest of all metals, making it ideal for use in electronics and thermal management applications. |
| Magnetic Susceptibility | Diamagnetic; gold is repelled by magnetic fields, although this effect is very weak. |
| Reflectivity | High; gold reflects infrared radiation, making it useful in protective coatings and heat management. |
| Color | Gold’s distinct yellow color is due to relativistic effects that affect the absorption of light. |
| Corrosion Resistance | Excellent; gold does not oxidize in air or water, preserving its electrical and thermal properties over time. |
| Nuclear Property | Description |
|---|---|
| Atomic Number (Z) | 79; gold has 79 protons in its nucleus. |
| Atomic Mass | Averages at about 196.966569 u; gold’s atomic mass is primarily due to its most stable isotope, ^197Au. |
| Isotopes | Gold has only one stable isotope, ^197Au. |
| Radioisotopes | Gold-198 (^198Au) is a commonly used radioisotope in medicine for cancer treatment. |
| Half-life of ^198Au | 2.7 days; ^198Au decays by beta decay into mercury. |
| Nuclear Spin of ^197Au | 3/2−; this property is useful in nuclear magnetic resonance (NMR) applications. |
| Cross Section for Neutron Capture | High; ^197Au has a high cross section for neutron capture, making it useful in nuclear reactors as a control material. |
old is predominantly obtained through mining operations as it naturally occurs in the Earth’s crust. It can be found either in its elemental (native) form, as nuggets or grains in rocks, and in alluvial deposits, or as a component of various minerals such as pyrite. The preparation of gold from ore involves several steps:
| Isotope | Half-life | Decay Mode | Notes |
|---|---|---|---|
| Au-194 | 1.588 days | Beta decay | Used for research and potential applications in nuclear medicine |
| Au-195 | Stable | N/A | Natural gold consists mostly of this isotope, used in studies of gold’s chemical behavior |
| Au-196 | 6.183 days | Electron capture | Has applications in nuclear science research |
| Au-197 | Stable | N/A | The only naturally occurring isotope, comprising the majority of gold found in nature |
| Au-198 | 2.69517 days | Beta decay | Used in medicine for cancer treatment, particularly in radiation therapy |
| Au-199 | 3.169 days | Beta decay | Investigated for use in nuclear medicine and research |
| Au-200 | 48.5 hours | Beta decay | Studied for its potential in medical and scientific research |
| Au-201 | 26 minutes | Beta decay | Has potential uses in nuclear medicine research |
| Au-202 | 28.6 hours | Beta decay | Of interest for its nuclear properties and potential applications in research |
The production of gold involves several key processes, from extraction and mining to refining and purification, to obtain pure gold from its natural sources:
Gold’s unique properties, including its conductivity, malleability, resistance to corrosion, and aesthetic appeal, make it valuable in a wide range of applications:
Gold’s timeless allure, combined with its unique physical and chemical properties, cements its status as a highly valued element across cultures and industries. From ancient artifacts to modern electronics and medical treatments, gold’s versatility and durability enable its widespread use. This exploration of gold, from its production to its myriad applications, highlights the element’s enduring significance and the innovative ways humanity continues to utilize this precious metal.
Text prompt
Add Tone
Chemical Properties of Gold
Thermodynamic Properties of Gold
What is the primary use of gold in modern industries?
Electronics
Pharmaceuticals
Food additives
Fuel additives
What characteristic of gold allows it to be shaped into extremely thin sheets?
Malleability
Elasticity
Brittleness
Hardness
Which property makes gold valuable for use in space equipment?
Reflectivity
Solubility
Flexibility
Magnetism
How is gold most commonly extracted from its ores?
Smelting
Electrolysis
Cyanidation
Filtration
Which historical period was marked by extensive gold mining and exploration in the Americas?
Bronze Age
Victorian Era
Gold Rush
Industrial Revolution
What is the unit of measurement typically used to weigh gold?
Pounds
Kilograms
Carats
Troy ounces
What environmental issue is often associated with gold mining?
Air pollution
Noise pollution
Water pollution
Light pollution
What is a common test used to verify the purity of gold?
Scratch test
Acid test
Heat test
Sound test
Which global market is the largest for gold trading?
London Bullion Market
New York Stock Exchange
Tokyo Commodity Exchange
Shanghai Gold Exchange
Before you leave, take our quick quiz to enhance your learning!

