What is the atomic number of hafnium?
72
73
74
75
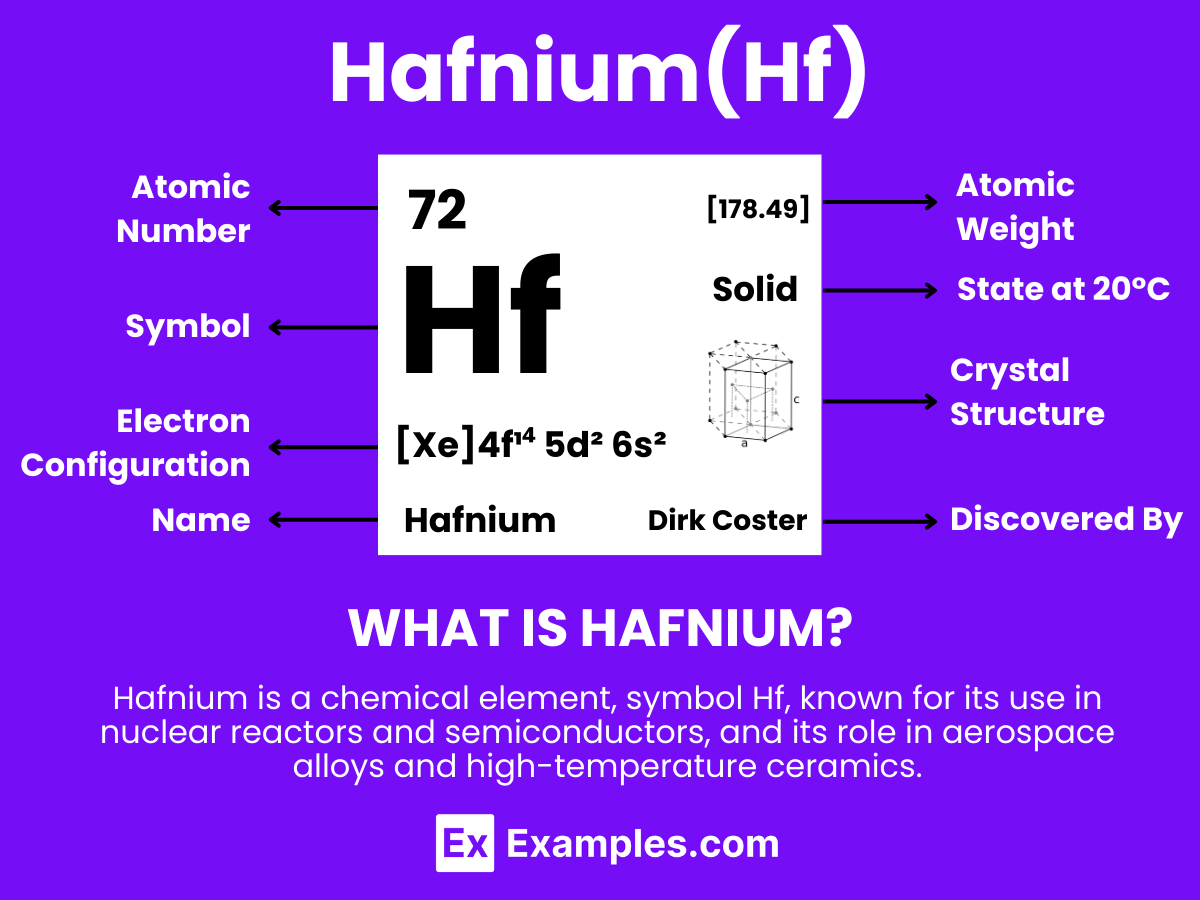
Hafnium, a lustrous, silver-gray transition metal, plays a pivotal role in modern technology and industry. This guide delves into the essence of hafnium, uncovering its unique properties, applications, and significance. Known for its remarkable resistance to corrosion and high melting point, hafnium is a key component in nuclear reactors, semiconductors, and aerospace engineering. Our exploration offers insights into hafnium’s characteristics, uses, and the latest advancements in research, providing a comprehensive understanding of this indispensable element. Discover how hafnium’s exceptional qualities contribute to advancements in technology and industry, making it a subject of increasing interest among scientists and engineers.
Hafnium is a metallic element with the chemical symbol Hf and atomic number 72. It is extracted from zirconium minerals, as it is commonly found in zirconium ores due to its similar chemical properties. Unlike lawrencium, hafnium exists naturally and is notable for its high melting point and strength at high temperatures, making it valuable in various industrial applications. The identification of hafnium was significant in the field of chemistry, particularly for its role in reinforcing the understanding of the periodic table’s transition metals. Due to its resistance to corrosion and ability to absorb neutrons, hafnium plays a crucial role in nuclear reactors, aerospace engineering, and electronics.
Formula: Hf
Composition: Comprises a single hafnium atom.
Bond Type: Hafnium readily forms bonds with other elements, including both covalent and ionic bonds. It is known for its ability to form complex compounds, particularly with oxygen, nitrogen, and carbon. The chemistry of hafnium is similar to that of zirconium, its group 4 counterpart, due to the lanthanide contraction.
Molecular Structure: In its elemental form, hafnium has a hexagonal close-packed structure. Hafnium’s compounds can have a variety of structures depending on the elements it bonds with. It is capable of forming complex oxides and carbides, which are essential in various industrial applications.
Electron Sharing: Hafnium participates in electron sharing to form covalent bonds and can also engage in ionic bonding, especially in its compounds such as hafnium dioxide (HfO₂) and hafnium carbide (HfC). These properties make hafnium compounds valuable for their high melting points and thermal stability.
Significance: Hafnium plays a crucial role in modern technology and materials science. It is used in the manufacturing of semiconductors, nuclear reactor control rods, and high-temperature alloys. Its ability to absorb neutrons makes it important for nuclear applications, while its high melting point and strength at elevated temperatures are valuable in aerospace applications.
Role in Chemistry: Hafnium’s role in chemistry extends beyond its basic atomic properties to include significant practical applications in electronics, nuclear technology, and materials science. Its chemical similarity to zirconium presents both challenges and opportunities in separation processes and in the study of their chemical behavior.
Understanding the atomic structure of Hafnium offers insights into its properties and applications. Possessing 72 protons in its nucleus.
The stability and phase of Hafnium under differing temperatures and pressures are well-documented, with hafnium existing as a solid under standard conditions. Its high melting point and strength at elevated temperatures make it valuable for use in high-temperature applications, such as aerospace components and nuclear reactor control rods.
| Property | Value |
|---|---|
| Appearance | Silvery gray, lustrous metallic |
| Atomic Number | 72 |
| Atomic Mass | 178.49 amu |
| State at 20 °C | Solid |
| Melting Point | 2233 °C |
| Boiling Point | 4603 °C |
| Density | 13.31 g/cm³ |
| Electron Configuration | [Xe]4f¹⁴ 5d² 6s² |
| Oxidation States | +4 (most stable), possible +2 |
| Crystal Structure | Hexagonal close-packed (hcp) |
Hafnium’s chemical properties are well-documented due to its stability and presence in various applications. Its chemistry is notable for its similarities to zirconium, making it challenging to separate these two elements:
| Property | Value for Hafnium |
|---|---|
| Melting Point | 2,233°C (4,051°F) |
| Boiling Point | 4,603°C (8,317°F) |
| Heat of Fusion | 25.73 kJ/mol |
| Heat of Vaporization | 630 kJ/mol |
| Specific Heat Capacity | 25.73 J/(mol·K) |
| Thermal Conductivity | 23 W/(m·K) |
| Thermal Expansion | 5.9 µm/(m·K) |
| Property | Value for Hafnium |
|---|---|
| Density | 13.31 g/cm³ at 20°C |
| Young’s Modulus | 78 GPa |
| Shear Modulus | 30 GPa |
| Bulk Modulus | 110 GPa |
| Poisson’s Ratio | 0.37 |
| Mohs Hardness | ~5.5 |
| Brinell Hardness | 1700 MPa |
| Property | Value for Hafnium |
|---|---|
| Electrical Resistivity | 331 nΩ·m (at 20°C) |
| Magnetic Susceptibility | +120.0·10⁻⁶ cm³/mol |
| Superconducting Point | Not Superconducting under normal conditions |
| Property | Value for Hafnium |
|---|---|
| Atomic Number | 72 |
| Atomic Weight | 178.49 |
| Isotopes | 176-Hf to 180-Hf (stable) |
| Neutron Cross Section | High for thermal neutrons |
| Neutron Absorption | Used in nuclear reactors as a control rod material |
Zirconium Separation: Hafnium is typically obtained as a by-product of zirconium refinement from zircon (ZrSiO₄) ores. The extraction process involves complex chemical separations due to the chemical similarity between zirconium and hafnium.
Liquid-Liquid Extraction: The separation of hafnium from zirconium is often achieved using liquid-liquid extraction techniques, employing organic solvents that preferentially bind to hafnium compounds.
Molten Salt Electrolysis: In some processes, hafnium is purified through molten salt electrolysis, where a mixture containing hafnium chloride is electrolyzed to produce pure hafnium metal.
Reduction Techniques: Pure hafnium is obtained by reducing hafnium chloride (HfCl₄) with magnesium (Mg) or sodium (Na), resulting in the production of hafnium metal.
Forms oxides, notably hafnium dioxide (HfO₂), used in semiconductor devices for its high dielectric constant and stability.
Equation: Hf + O₂ → HfO₂
Tetrafluoride (HfF₄) is produced in reactions involving hafnium and fluorine, showcasing hafnium’s ability to form compounds in a +4 oxidation state.
Equation: Hf + 2F₂ → HfF₄
Tetrachloride (HfCl₄) is synthesized through the direct chlorination of hafnium, illustrating its reactivity with halogens.
Equation: Hf + 2Cl₂ → HfCl₄
Tetraiodide (HfI₄) can be produced, indicating hafnium’s potential to achieve a +4 oxidation state with iodine.
Equation: Hf + 2I₂ → HfI₄
In aqueous solutions, hafnium can form various ions and complexes, highlighting its chemical versatility.
Equation: Hf → Hf₄⁺ + 4e⁻
Forms complex ions with different ligands, underlining its role in coordination chemistry.
Equation: Hf₄⁺ + nL → [HfLn]₄⁺
| Isotope | Half-life | Mode of Decay |
|---|---|---|
| Hafnium-253 | 0.6 seconds | Alpha decay |
| Hafnium-254 | 13 seconds | Alpha decay |
| Hafnium-255 | 21.5 seconds | Alpha decay |
| Hafnium-256 | 27 seconds | Alpha decay |
| Hafnium-257 | 0.65 seconds | Alpha decay |
| Hafnium-258 | 4.1 seconds | Alpha decay |
| Hafnium-259 | 6.2 seconds | Alpha decay |
| Hafnium-260 | 2.7 minutes | Alpha decay |
Extraction from Zirconium Ores: Hafnium is extracted from minerals like zircon (ZrSiO4) due to its presence in nearly all zirconium-bearing ores. The extraction process involves separating hafnium from zirconium, given their chemical and physical similarities.
Separation Process: The primary method for hafnium production involves liquid-liquid extraction or the use of ion-exchange chromatography. These processes separate hafnium from zirconium based on their differential solubilities or ion-exchange properties.
Refining Techniques: After initial separation, hafnium is further refined through processes like reduction or van Arkel-de Boer process, resulting in high-purity hafnium metal or compounds suitable for various applications.
Zirconium Co-production: The production of hafnium is often a byproduct of zirconium refinement, where the focus is on producing zirconium for industrial use, and hafnium is extracted from the zirconium processing stream.
Handling and Safety: Special care is required in handling hafnium, particularly in its powder form, due to its ability to absorb hydrogen and oxidize at elevated temperatures, posing potential safety risks.
Analytical Detection: Methods such as X-ray fluorescence (XRF) and mass spectrometry are utilized to quantify hafnium concentrations, ensuring the effectiveness of the separation process and the purity of the final product.
Nuclear Reactors: Hafnium’s excellent neutron-absorption capabilities make it a critical component in nuclear reactor control rods, enhancing safety and efficiency in nuclear power generation.
Aerospace Engineering: The high melting point and strength of hafnium at elevated temperatures lend it to applications in aerospace components, including jet engines and space vehicles.
Semiconductors: Hafnium oxide is used in the production of semiconductors, improving the performance of electronic devices by allowing for smaller, more efficient transistors.
Alloy Additive: Hafnium is added to various alloys to improve their mechanical properties, corrosion resistance, and temperature stability, useful in applications ranging from chemical processing to high-temperature environments.
Research and Development: Beyond industrial applications, hafnium is a subject of research, particularly in materials science, where its unique properties can lead to new discoveries and technologies.
Advanced Electronics: Its role in advanced computing and electronics continues to grow, particularly with the development of hafnium-based compounds for next-generation electronic devices.
This comprehensive overview of Hafnium covers its production methods and wide-ranging applications in industry and technology. As a naturally occurring element, hafnium plays a significant role in enhancing the performance and safety of nuclear reactors, contributing to advances in aerospace and semiconductor technologies, and offering valuable insights in materials science research. Its versatility and unique properties underscore hafnium’s importance in both practical applications and the exploration of material science frontiers.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of hafnium?
72
73
74
75
Hafnium is classified under which group in the periodic table?
Alkali metals
Transition metals
Halogens
Noble gases
Which of the following is the chemical symbol for hafnium?
Hf
Ha
Hm
Hn
What is the primary use of hafnium in industry?
Jewelry
Nuclear reactors
Fertilizers
Batteries
Hafnium is often found in ores with which other element?
Titanium
Zirconium
Iron
Lead
What is the melting point of hafnium?
1660°C
1855°C
2233°C
2506°C
Hafnium is resistant to which type of corrosion?
Water
Alkali
Acid
Oxidation
What color is hafnium in its pure form?
Silver
Gold
Black
Blue
Which property makes hafnium useful in high-temperature applications?
High thermal conductivity
Low melting point
Low density
High neutron capture cross-section
Hafnium is used in the manufacturing of which electronic component?
Transistors
Resistors
Capacitors
Inductors
Before you leave, take our quick quiz to enhance your learning!

