Lawrencium (Lr) – Definition, Preparation, Properties, Uses, Compounds, Reactivity
Dive into the enigmatic world of Lawrencium, an element shrouded in mystery and intrigue. This guide offers a comprehensive exploration of Lawrencium, shedding light on its definition, significance, and the unique role it plays in the realm of nuclear chemistry. Despite its scarcity and the challenges associated with its study, Lawrencium captures the curiosity of scientists and scholars alike. From its theoretical uses to the complex compounds it forms, this element continues to push the boundaries of our scientific understanding. Embark on this fascinating journey to discover the secrets and potential applications of Lawrencium, and witness how this superheavy element contributes to advancing our knowledge of the periodic table and the fundamental forces that govern the atomic world.
What is Lawrencium?
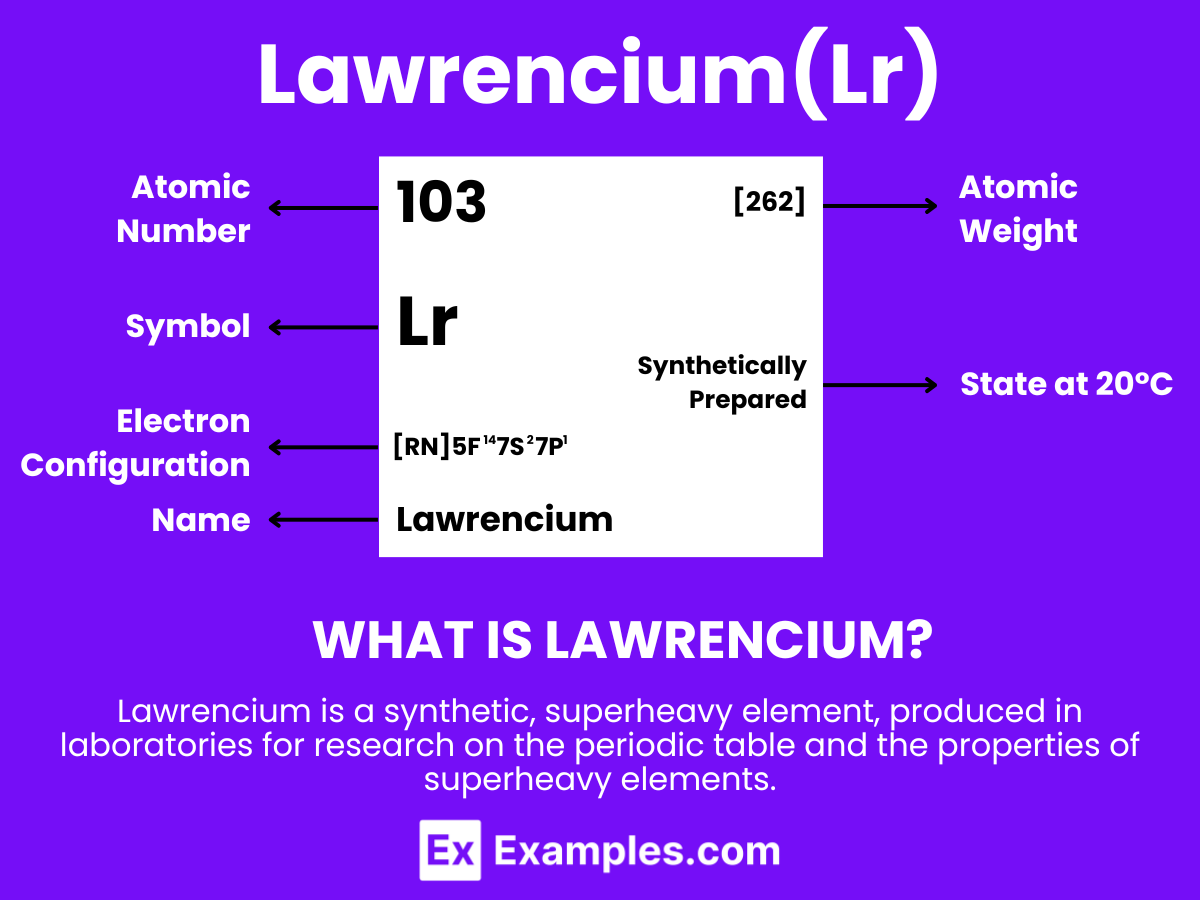
Lawrencium is a synthetic element with the chemical symbol Lr and atomic number 103. It is produced in particle accelerators through the bombardment of lighter atomic nuclei. Like nobelium, lawrencium does not exist naturally and is known for its extremely short half-life, which limits the opportunity for detailed study. The discovery of lawrencium is important for the field of nuclear physics, particularly in the examination of the properties of superheavy elements in the periodic table’s actinide series. Due to its radioactivity and instability, lawrencium’s use is restricted to research purposes, aiding in the expansion of knowledge concerning the behavior of heavy elements and exploring the theoretical boundaries of the periodic table.
Lawrencium Formula
Formula: Lr
Composition: Comprises a single lawrencium atom.
Bond Type: As a highly radioactive synthetic element, lawrencium is not known to form bonds in its most stable isotopes due to its short half-life. While theoretical predictions suggest the possibility of covalent or ionic bonding in compounds, actual chemical behavior is mostly uncharted because it exists only in trace amounts and for very brief moments. Molecular Structure: In its elemental form, lawrencium does not have a defined molecular structure. Similar to other heavy and short-lived elements, any potential molecular interactions or solid-state behaviors of lawrencium remain theoretical.
Electron Sharing: Lawrencium could theoretically participate in electron-sharing to form covalent bonds or could engage in ionic bonding under certain conditions. Nevertheless, the synthesis of stable compounds involving lawrencium is hindered by its instability, making any chemical properties of lawrencium speculative. Significance: The significance of lawrencium lies in its role in scientific research, contributing to our understanding of the actinide series and superheavy elements. Despite the obstacles in its production and the minute quantities obtained, investigating lawrencium pushes the boundaries of knowledge regarding the periodic table and the characteristics of transuranium elements.
Role in Chemistry: Lawrencium’s role in chemistry is predominantly experimental and hypothetical. Its potential to shed light on the chemical behavior of actinides is significant, though practical chemical applications are absent due to the element’s extreme radioactivity, the challenge of its production, and the short duration of observations allowed by its fleeting existence.
Atomic Structure of Lawrencium
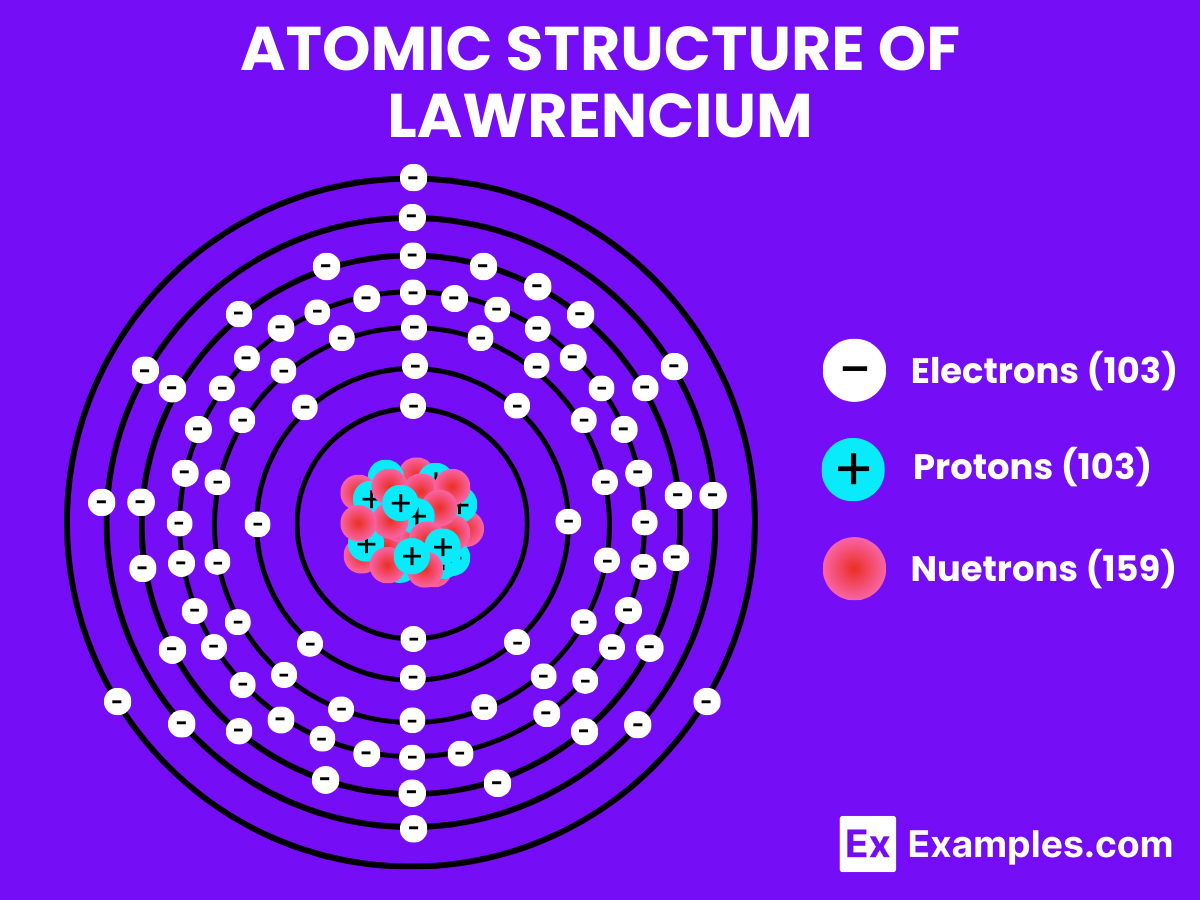
Understanding the atomic structure of Lawrencium offers intriguing insights into its distinctive role within the periodic table and the field of nuclear chemistry. Possessing 103 protons in its nucleus.
Atomic Level: Each atom of Lawrencium (Lr) is defined by the presence of 103 protons in its nucleus, which establishes its atomic number as 103. The theoretical electron configuration for Lawrencium is [Rn]5f¹⁴ 7s² 7p¹, suggesting a complete 5f orbital, two electrons in the 7s orbital, and one electron in the 7p orbital, which forms the basis for potential chemical interactions.
Molecular Formation: Lawrencium, much like other superheavy elements, does not naturally form stable molecules or exhibit a consistent molecular structure due to its incredibly short half-life and pronounced instability. This element exists momentarily before undergoing decay into lighter elements.
The stability and phase of Lawrencium under differing temperatures and pressures remain a matter of theoretical conjecture, as its transient nature makes it impossible to observe its state as solid, liquid, or gas under standard conditions.
Properties of Lawrencium
Physical Properties of Lawrencium
| Property | Value |
|---|---|
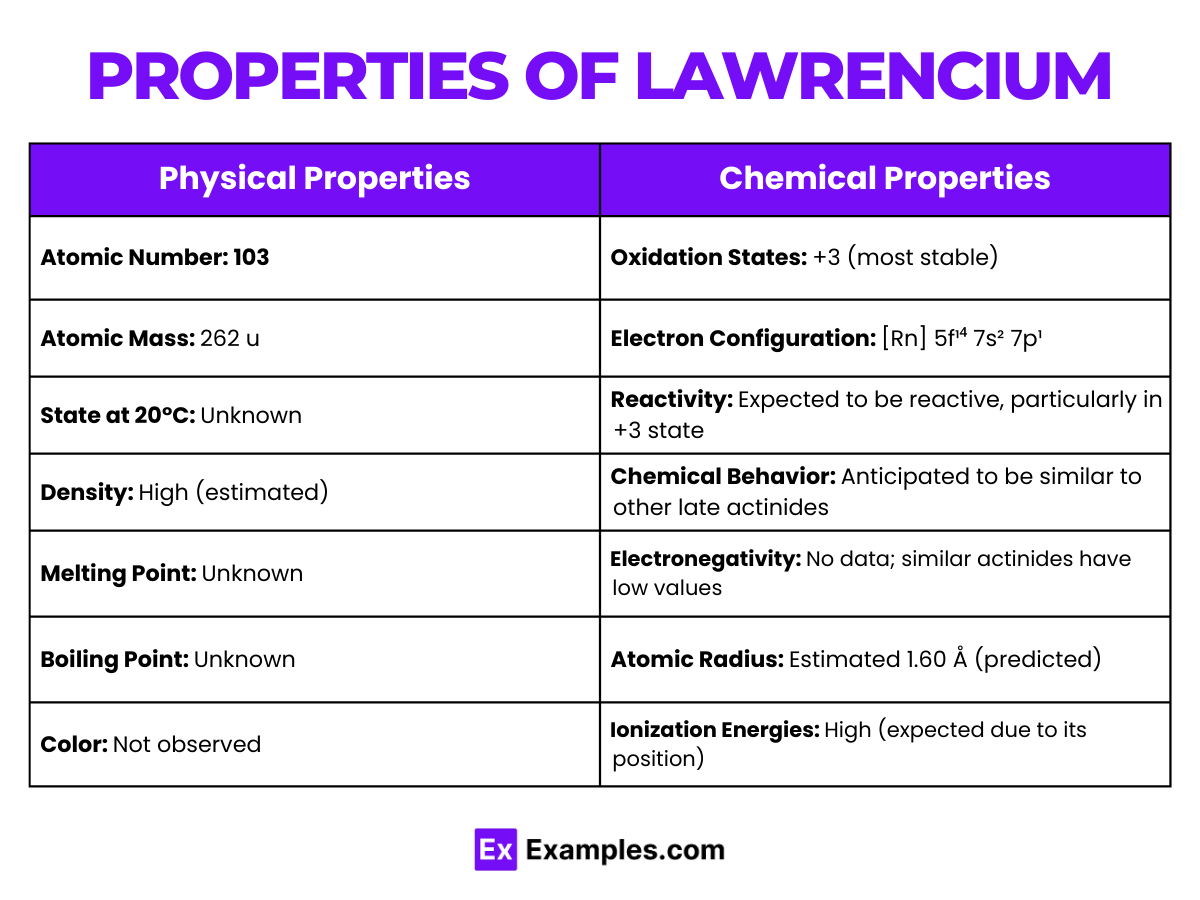
| Appearance | Unknown, presumably metallic |
| Atomic Number | 103 |
| Atomic Mass | (262)amu |
| State at 20 °C | Solid (predicted) |
| Melting Point | Not accurately known; predicted to be around 1627 °C |
| Boiling Point | Not determined |
| Density | Estimated to be around 15.6 – 16.6 g/cm³ (predicted) |
| Electron Configuration | [Rn] 5f¹⁴ 7s² 7p¹(predicted) |
| Oxidation States | +3 (most stable), possible +1 |
| Crystal Structure | Unknown |
Chemical Properties of Lawrencium
Lawrencium’s chemical properties are primarily theoretical due to its short half-life and the challenges of producing it in significant quantities. Its chemistry is expected to be similar to other actinides, with a few notable predictions:
- Oxidation States:
- Lawrencium predominantly exhibits a +3 oxidation state, similar to other late actinides. The presence of a 7p electron suggests a possible +1 oxidation state, but this is less stable and not commonly observed.
- Halides Formation:
- Like other actinides, lawrencium is expected to form halides, such as LrF₃, LrCl₃, LrBr₃, and LrI₃.
- Oxide Formation:
- Lawrencium oxide (Lr2O₃) formation is anticipated, aligning with its +3 oxidation state.
- Aqueous Chemistry:
- In aqueous solutions, lawrencium is expected to form the Lr₃+ ion. Its behavior in water and with other aqueous compounds would provide insights into its chemical reactivity and coordination chemistry.
- Complex Formation:
- The potential for lawrencium to form various complexes, especially in its +3 oxidation state, is anticipated. Research into these areas could unveil unique aspects of lawrencium’s chemistry compared to other actinides.
Nuclear Properties of Lawrencium
| Property | Value for Lawrencium |
|---|---|
| Atomic Number | 103 |
| Atomic Mass | 262 u (most stable isotope Lawrencium-262) |
| Radioactive | Yes, all isotopes have short half-lives |
| Oxidation States | +3 (most stable and common for Lawrencium) |
| Reactivity | Reactive, particularly in its +3 oxidation state |
| Ionization Energies | High |
| Compounds | Few known, Lawrencium is mostly studied in its elemental form due to instability |
Preparation of Lawrencium
Particle Accelerator Bombardment: Lawrencium is typically produced by bombarding californium (Cf) isotopes with boron (B) or nitrogen (N) ions using a particle accelerator. These high-energy collisions lead to the fusion of atomic nuclei.
Isotope Targeting: Different isotopes of Lawrencium can be synthesized by adjusting the isotopes of californium used as targets and the type of ions in the bombardment process.
Alpha-particle Bombardment: An alternative method for producing Lawrencium involves the bombardment of heavier actinide targets, like einsteinium, with alpha particles (helium nuclei).
Electron Capture Following Bombardment: Post-bombardment, some isotopes might undergo beta decay, where a neutron within the nucleus is transformed into a proton, thereby facilitating the formation of Lawrencium isotopes through electron capture.
Chemical Separation: After the bombardment process, chemical techniques are applied to separate Lawrencium from other elements and isotopes.
Identification by Decay Patterns: The identification of Lawrencium is verified by observing its decay patterns, primarily through alpha decay, and comparing these observations with established data.
Chemical Compounds of Lawrencium
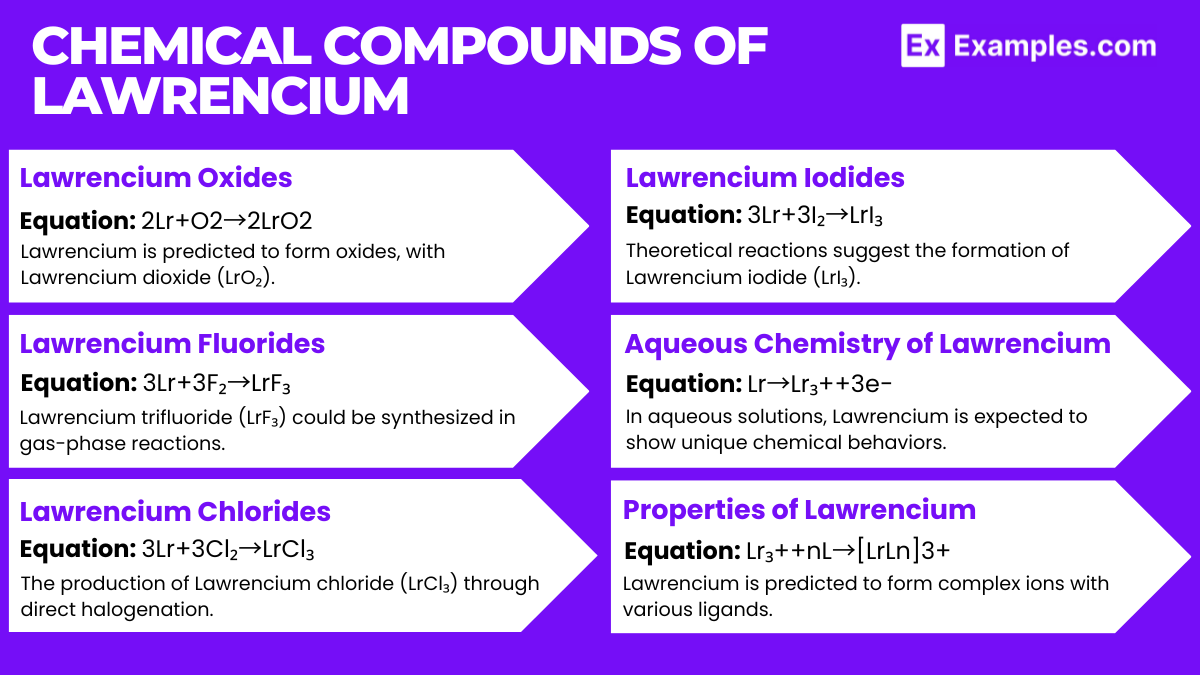
Lawrencium Oxides
- Lawrencium is predicted to form oxides, with Lawrencium dioxide (LrO₂) being a theoretical compound due to its position in the actinide series.
- Equation: 2Lr+O2→2LrO2
Lawrencium Fluorides
- Lawrencium trifluoride (LrF₃) could be synthesized in gas-phase reactions, suggesting Lawrencium’s capability to form compounds in a +3 oxidation state, consistent with actinide chemistry.
- Equation: 3Lr+3F₂→LrF₃
Lawrencium Chlorides
- The production of Lawrencium chloride (LrCl₃) through direct halogenation would indicate Lawrencium’s reactivity with halogens.
- Equation: 3Lr+3Cl₂→LrCl₃
Lawrencium Iodides
- Theoretical reactions suggest the formation of Lawrencium iodide (LrI₃), demonstrating the element’s potential to exhibit the +3 oxidation state with iodine.
- Equation: 3Lr+3I₂→LrI₃
Aqueous Chemistry of Lawrencium
- In aqueous solutions, Lawrencium is expected to show unique chemical behaviors, likely forming ions such as Lr³⁺.
- Equation: Lr→Lr₃++3e−
Complex Formation
- Lawrencium is predicted to form complex ions with various ligands, highlighting its potential involvement in coordination chemistry.
- Equation: Lr₃++nL→[LrLn]3+
Isotopes of Lawrencium
| Isotope | Half-life | Mode of Decay |
|---|---|---|
| Lawrencium-253 | 0.6 seconds | Alpha decay |
| Lawrencium-254 | 13 seconds | Alpha decay |
| Lawrencium-255 | 21.5 seconds | Alpha decay |
| Lawrencium-256 | 27 seconds | Alpha decay |
| Lawrencium-257 | 0.65 seconds | Alpha decay |
| Lawrencium-258 | 4.1 seconds | Alpha decay |
| Lawrencium-259 | 6.2 seconds | Alpha decay |
| Lawrencium-260 | 2.7 minutes | Alpha decay |
Uses of Lawrencium
- Frontiers in Nuclear Science: Lawrencium’s study is pivotal in advancing our comprehension of nuclear chemistry and physics, offering insights into the behavior and stability of superheavy elements, which are crucial for refining theoretical models of atomic nuclei.
- Exploring Atomic Realms: Research on Lawrencium sheds light on the behavior of electrons in the orbitals of heavy elements, providing valuable data on relativistic effects impacting atomic and molecular structures.
- Chemistry of the Unknown: Investigating Lawrencium helps unravel the complex chemical properties of the actinide series, enhancing our understanding of periodic trends and element reactivity within this elusive group.
- Pathway to Discovery: The synthesis techniques developed for Lawrencium research aid in the discovery of new elements, expanding the periodic table and fostering developments in nuclear technology.
- Medical Innovations: While not directly used in medical treatments, the study of Lawrencium’s radioactive properties supports the development of advanced cancer treatment methods, such as targeted alpha therapy (TAT), aiming to minimize damage to healthy tissues.
- Educational Inspiration: The story of Lawrencium—from synthesis challenges to its role in scientific discovery—provides a compelling narrative for science education, sparking interest and inspiration among students exploring the periodic table’s evolution.
- Precision Instrumentation: In specialized research settings, Lawrencium isotopes may help calibrate advanced instruments for radioactive material detection, underlining its importance in scientific measurement and analysis.
- Pushing Scientific Boundaries: Pursuing knowledge of Lawrencium challenges existing scientific theories, encouraging deeper exploration into the fundamental components of the universe.
Production of Lawrencium
Particle Accelerators: Lawrencium is synthesized in particle accelerators by bombarding target materials like californium with light ions, such as boron or carbon, under high-energy conditions.
Bombardment Process: The creation of Lawrencium involves high-energy collisions, where target atoms are bombarded with ions, leading to the formation of Lawrencium isotopes after absorbing the kinetic energy and undergoing nuclear fusion.
Nuclear Reaction: A typical reaction for producing Lawrencium might involve the collision of californium-249 with boron-11 ions, showcasing the intricate process of fusing different nuclei to create superheavy elements.
Isolation Techniques: Following synthesis, Lawrencium is isolated through chemical separations, such as liquid-liquid extraction or ion-exchange chromatography, due to its radioactivity and the need for precise handling.
Radioactivity Handling: Specialized containment and safety procedures are necessary to manage Lawrencium’s radioactivity, ensuring the well-being of researchers and the integrity of experimental outcomes.
Analytical Detection: Techniques like alpha spectrometry are employed to identify Lawrencium, allowing scientists to confirm its production by detecting characteristic radiation signatures.
Applications of Lawrencium
Scientific Research: The primary application of Lawrencium lies in fundamental research, particularly in studying the properties and decay processes of superheavy elements, thereby enriching our knowledge of the actinide series.
Nuclear Physics: Lawrencium research contributes to nuclear physics by exploring the stability of superheavy nuclei, investigating shell effects, and theorizing about the “island of stability.”
Heavy Element Chemistry: Studies involving Lawrencium extend the boundaries of inorganic chemistry, offering insights into oxidation states, electronic structures, and the potential for forming compounds.
Synthesis of Heavier Elements: As a precursor in the synthesis of heavier elements, Lawrencium plays a critical role in discovering new periodic table members through successive nuclear reactions.
Educational Resource: Although not used in standard educational settings, the discovery and characteristics of Lawrencium serve as valuable teaching materials for advanced science courses, inspiring future generations of scientists.
Specialized Research: Lawrencium isotopes may be utilized in niche scientific investigations, such as probing the limits of atomic number behaviors and element stability, highlighting its significance in pushing the frontiers of scientific inquiry.
This article thoroughly investigated Lawrencium, highlighting its isotopes, production methods, and applications in nuclear science and beyond. As a synthetic, superheavy element, Lawrencium challenges our understanding of chemical and nuclear physics, offering a window into the behavior of the periodic table’s most elusive members. Its study not only advances scientific knowledge but also inspires future explorations into the unknown realms of the atomic world.