Nylon is a type of
Natural polymer
Synthetic polymer
Semi-synthetic polymer
Monomer


Nylon is an important chemistry invention, known as a synthetic polymer, which means it’s a man-made material created through a series of chemical reactions. It belongs to the family of organic compounds, which are substances primarily made of carbon and hydrogen. Nylon is strong, flexible, and resistant to wear and tear, making it useful for a variety of applications such as clothing, ropes, and even toothbrush bristles. Its versatility and durability make it a valuable material in our everyday lives
This type is made from a single type of monomer called caprolactam and is known for its strength and stiffness. It is commonly used in fibers for carpets and clothing.
Made from two monomers, adipic acid and hexamethylenediamine, Nylon 6,6 is famous for its excellent wear resistance and mechanical strength. It’s often found in high-quality gears, bearings, and car parts.
These types are known for their flexibility and resistance to impact, making them ideal for products that need to absorb shock without breaking, like fuel lines and pneumatic air tubes.
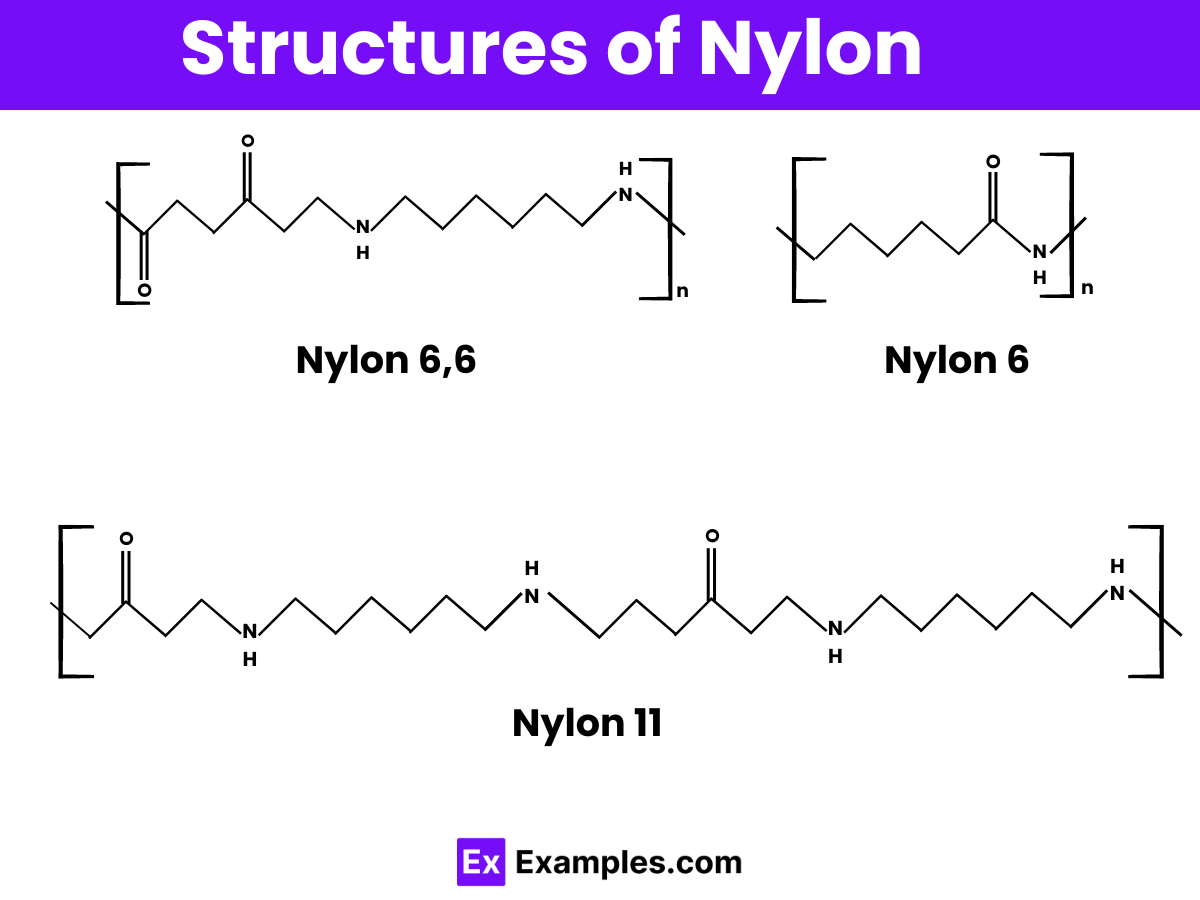
Nylon’s structure is unique and fascinating, consisting of long chains of molecules called polymers. These polymers are made through a chemical reaction known as polymerization, where smaller molecules called monomers link together to form a large chain. For example, in Nylon 6,6, the monomers hexamethylenediamine and adipic acid combine to create repeating units that give Nylon its strength and flexibility. This structure allows Nylon to be incredibly durable and resistant to wear and tear, making it ideal for various applications such as textiles, machinery parts, and even automotive components. The arrangement of these molecules is what makes Nylon so versatile and valuable in many industries.
Nylon is typically created through a fascinating chemical process called polycondensation. One of the most common methods to make Nylon involves reacting two specific types of molecules: a dicarboxylic acid and a diamine. For instance, in the production of Nylon 6,6, the dicarboxylic acid adipic acid reacts with the diamine hexamethylenediamine.
During the reaction, water molecules are released as byproducts, and the acid and diamine combine to form long, chain-like molecules or polymers. This process is an example of a condensation reaction because it joins two molecules together while eliminating a smaller molecule (water). Here is the basic chemical equation for the formation of Nylon 6,6:
This equation shows how the adipic acid and hexamethylenediamine repeat units link together to form the nylon polymer, with water being the byproduct of each linkage. This method of synthesis allows Nylon to have very customizable properties, depending on how it’s processed, which makes it suitable for a wide range of applications from everyday items to industrial products.
| Property | Description |
|---|---|
| Strength | Nylon is very strong and can withstand significant stress before breaking. |
| Elasticity | It has good elasticity, allowing it to stretch and return to its original shape without deforming. |
| Abrasion Resistance | Nylon is highly resistant to wear and tear, making it ideal for products that undergo frequent friction. |
| Melting Point | It has a high melting point, typically around 220°C (428°F), which allows it to be used in high-temperature environments. |
| Moisture Absorbency | Nylon absorbs more moisture than some other polymers, which can affect its strength and durability. |
| Thermal Stability | It maintains its properties well under a range of temperatures, making it suitable for various climates and conditions. |
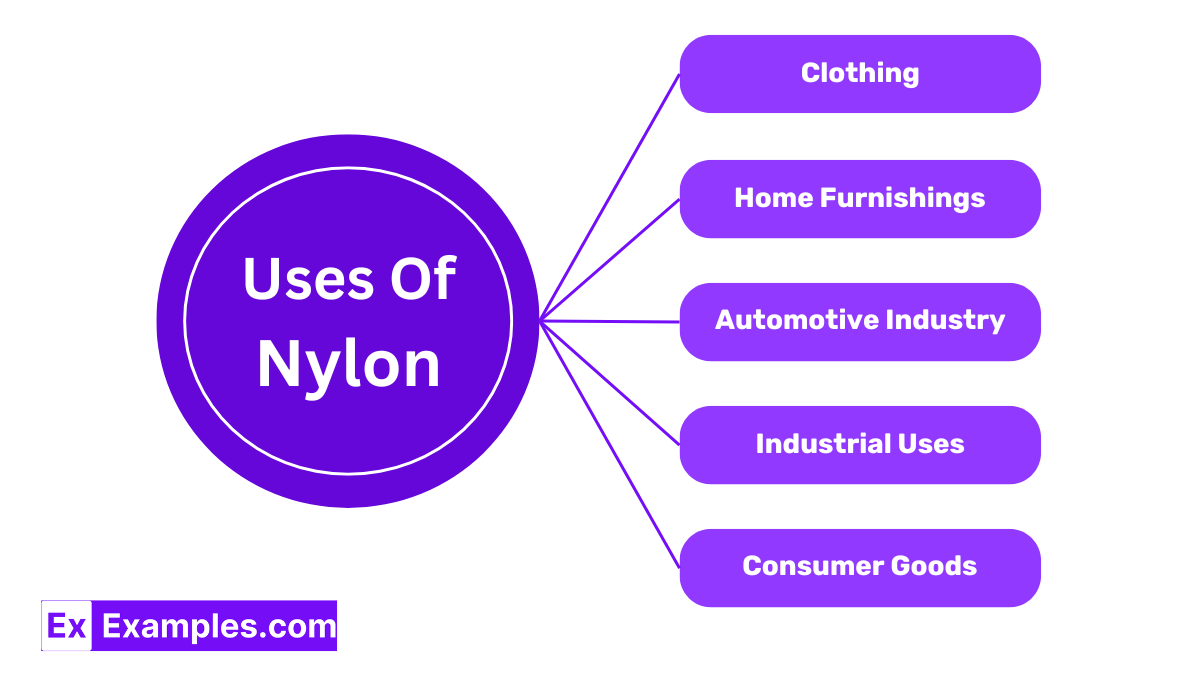
Nylon is extensively used in the fashion industry, especially for making hosiery and swimwear. Its elasticity and resistance to mildew make it an ideal choice for these applications. Additionally, it is used in the production of outerwear due to its ability to block wind and retain heat.
In homes, nylon is popular in carpet manufacturing because of its durability and resistance to stains and abrasion. It is also used in upholstery fabrics where high wear resistance is needed.
Nylon is a key material in the automotive sector, used in the manufacturing of car components like seat belts, airbags, tires, and various engine parts. Its heat resistance and durability contribute to safety and longevity.
Due to its strength and flexibility, nylon is used in making various sports equipment such as racquet strings, fishing lines, and nets. It withstands repeated stress and exposure to different weather conditions, making it perfect for outdoor sports gear.
Nylon’s mechanical strength and thermal stability make it suitable for mechanical parts such as gears, bearings, and conveyor belts. It is used in these applications because it can handle heavy loads and high temperatures without losing its shape or functionality.
Everyday items such as toothbrush bristles, food packaging, and kitchen utensils often contain nylon. Its safety for contact with food and resistance to microbial growth enhances the utility and hygiene of these products.
Nylon is a synthetic polymer, known for its strength, flexibility, and resistance to chemicals and wear.
Nylon is considered a good material due to its durability, versatility, and excellent resistance properties.
Nylon is a type of plastic used widely as a fabric in textiles due to its fiber-like properties.
Yes, nylon is tougher than cotton, offering superior strength and durability, especially in challenging environments.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Nylon is a type of
Natural polymer
Synthetic polymer
Semi-synthetic polymer
Monomer
Nylon is primarily used in the production of
Clothing and textiles
Glass
Wood products
Plastics
What is the primary raw material used to produce Nylon?
Coal
Natural rubber
Petroleum
Wood
Nylon is an example of
Natural polymer
Addition polymer
Cross-linked polymer
ondensation polymer
Which property of Nylon makes it suitable for use in fishing lines?
High density
High tensile strength
Low flexibility
High thermal conductivity
What is the main difference between Nylon 6 and Nylon 6,6?
Number of carbon atoms in the monomer units
Flexibility
Weight
Melting point
Which of the following is not a property of Nylon?
Water absorbent
Lightweight
Resistant to abrasion
Elastic
Nylon was first commercially used for which product?
Parachutes
Stockings
Ropes
Tires
Which process is used to make Nylon fibers?
Extrusion
Spinning
Casting
Molding
What type of chemical bond holds the monomers in Nylon together?
Ionic bond
Hydrogen bond
Covalent bond
Metallic bond
Before you leave, take our quick quiz to enhance your learning!

