What is the atomic number of promethium?
60
61
62
63

Dive into the enigmatic world of Promethium, a rare and fascinating element nestled within the lanthanide series of the periodic table. This comprehensive guide unveils Promethium’s unique characteristics, shedding light on its elusive nature, intriguing uses, and the compelling science behind its compounds. From enhancing the luminosity in watches to powering space exploration devices, Promethium’s applications are as diverse as they are remarkable. Embark on a journey to explore how this scarcely found element impacts technology, science, and beyond, enriched with examples that bring its story to life.
Promethium is a chemical element with the symbol Pm and atomic number 61, making it one of the rare earth elements within the lanthanide series of the periodic table. It is unique among the lanthanides as it does not occur in significant amounts in the earth’s crust in a natural, stable form due to its highly radioactive nature. All its isotopes are radioactive, with promethium-145 being the most stable, having a half-life of 17.7 years.Discovered in 1945 by Jacob A. Marinsky, Lawrence E. Glendenin, and Charles D. Coryell, the element was named after Prometheus, the Titan from Greek mythology who stole fire from the gods and gave it to humanity. The name reflects the discovery of the element amidst the development of nuclear technology and the atomic bomb in the mid-20th century.
Formula: Pm
Composition: Comprised solely of promethium atoms, making it an elemental substance.
Bond Type: As an element, promethium does not form bonds in its pure state. However, it can form various types of chemical bonds, such as ionic and covalent bonds, when it reacts with other elements. This ability enables promethium to create a multitude of compounds.
Molecular Structure: In its elemental form, promethium doesn’t exhibit a molecular structure. It assumes a metallic structure, likely adopting a hexagonal close-packed crystalline form, indicative of its properties as a potentially lustrous, silvery metal, although as a radioactive element, it is not commonly observed in bulk metallic form.
Electron Sharing: Promethium can share electrons to form covalent bonds or transfer electrons to form ionic bonds. It commonly assumes a +3 oxidation state (Pm³⁺) in its compounds, contributing to its versatility in forming various chemical species.
Significance: Promethium’s importance is notable in niche applications due to its radioactive nature. It is used in beta voltaic nuclear batteries, luminous paint, and as a light source in signaling equipment. Despite its limited availability and specific uses, promethium’s potential in applications like space exploration and portable energy sources illustrates its unique value.
Role in Chemistry: Promethium’s chemical behavior is intriguing within the study of the lanthanide series, underscoring the complexity of rare earth elements. Its radioactive properties and potential for electron exchange make it an interesting subject for theoretical and applied chemistry research, contributing to our knowledge of rare earth metals’ behavior, particularly in how radioactivity influences chemical interactions and technological applications.
Promethium is a rare earth metal that belongs to the lanthanide series of the periodic table. It is defined by its atomic number 61, meaning it has 61 protons in its nucleus. The atomic structure of promethium is characterized by the following:
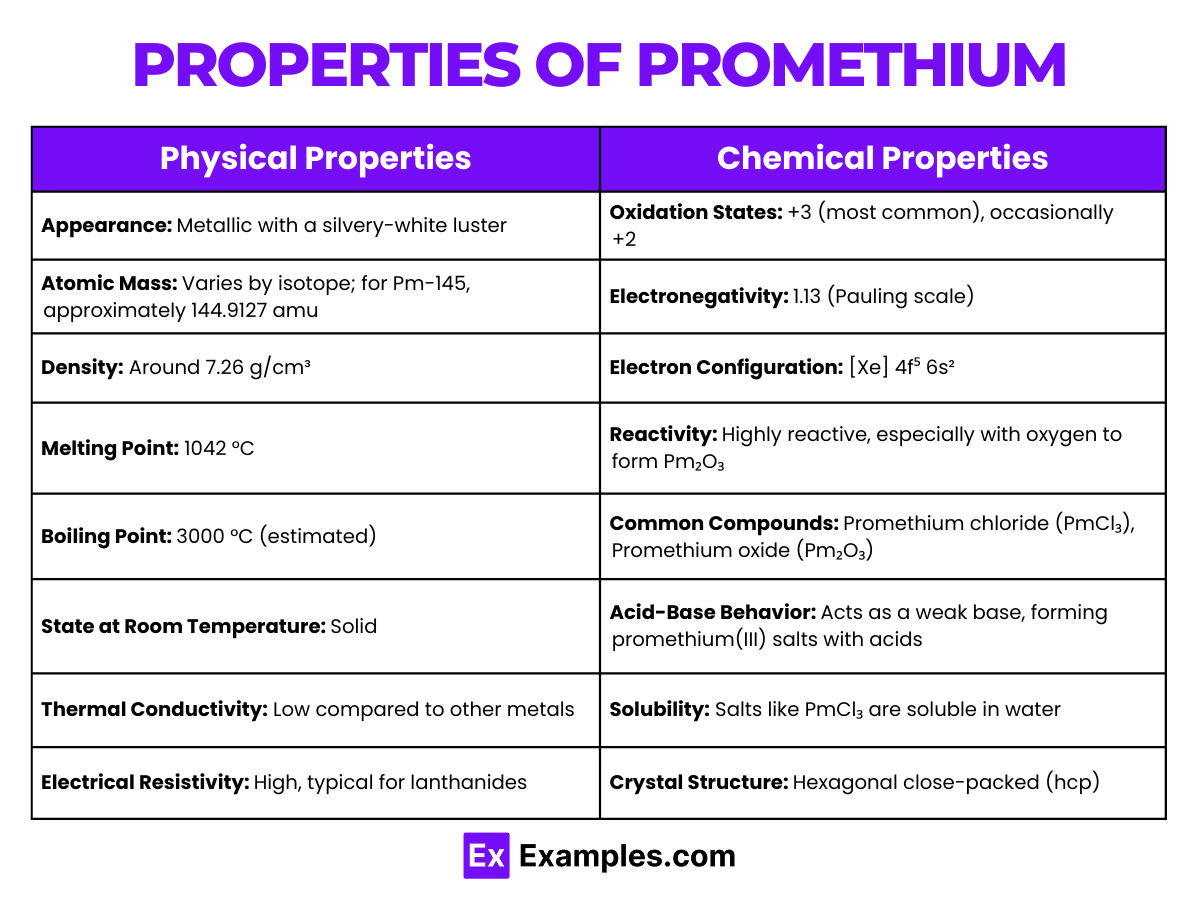
| Property | Value |
|---|---|
| Appearance | Metallic, silvery-white luster |
| Atomic Mass | Varies by isotope; for Pm-145, approximately 144.9127 amu |
| Density | About 7.26 g/cm³ at room temperature |
| Melting Point | 1042 °C |
| Boiling Point | Estimated 3000 °C |
| State at Room Temperature | Solid |
| Thermal Conductivity | Lower than most metals |
| Electrical Resistivity | High, characteristic of lanthanides |
| Magnetic Ordering | Paramagnetic at room temperature |
Promethium, symbolized as Pm and atomic number 61, exhibits several chemical properties that reflect its position in the lanthanide series of the periodic table. Its chemical behavior is characterized by the following aspects:
| Property | Value |
|---|---|
| Melting Point | 1042 °C |
| Boiling Point | Estimated 3000 °C |
| Heat of Fusion | Estimated 7.7 kJ/mol |
| Heat of Vaporization | Estimated 290 kJ/mol |
| Specific Heat Capacity | Estimated 100 J/(kg·K) |
| Thermal Conductivity | Low, specific value not provided |
| Thermal Expansion | Estimated, similar to lanthanides |
| Property | Value |
|---|---|
| Density | 7.26 g/cm³ at room temperature |
| Mohs Hardness | Similar to lanthanides, estimated around 2.5 |
| Young’s Modulus | Not specifically known for Promethium |
| Shear Modulus | Not specifically known for Promethium |
| Bulk Modulus | Not specifically known for Promethium |
| Poisson’s Ratio | Similar to lanthanides, not specifically provided for Promethium |
| Brinell Hardness | Not specifically known for Promethium |
| Property | Value |
|---|---|
| Electrical Resistivity | High, specific value not provided |
| Magnetic Ordering | Paramagnetic at room temperature |
| Curie Temperature | Not applicable to paramagnets |
| Superconducting Point | Promethium is not known to superconduct |
| Property | Value |
|---|---|
| Natural Isotopes | None, all isotopes are synthetic |
| Most Stable Isotope | Pm-145 with a half-life of 17.7 years |
| Neutron Cross Section | Varies with isotope, significant for Pm-149 |
| Neutron Mass Absorption | Specific to isotope, important for nuclear applications |
| Isotopic Abundance | Not naturally occurring, produced in nuclear reactors |
Promethium is a unique element in that it does not naturally occur in significant amounts on Earth due to its radioactivity. All its isotopes are radioactive, with promethium-145 (Pm-145) being the most stable but still with a half-life of only about 17.7 years. The preparation of promethium primarily involves nuclear reactions, typically occurring within nuclear reactors or during the processing of nuclear materials. Here are the key steps involved in the preparation of promethium:
1.Promethium Oxide (Pm₂O₃)
2.Promethium Fluoride (PmF₃)
3.Promethium Chloride (PmCl₃)
4.Promethium Sulfate (Pm₂(SO₄)₃)
5.Promethium Carbonate (Pm₂(CO₃)₃)
6.Promethium Hydroxide (Pm(OH)₃)
| Isotope | Mass Number | Half-Life | Decay Mode |
|---|---|---|---|
| Pm-143 | 143 | 265 days | Beta decay to Nd-143 |
| Pm-144 | 144 | 363 days | Beta decay to Nd-144 |
| Pm-145 | 145 | 17.7 years | Beta decay to Nd-145 |
| Pm-146 | 146 | 5.53 years | Beta decay to Nd-146 |
| Pm-147 | 147 | 2.62 years | Beta decay to Sm-147 |
| Pm-148 | 148 | 5.368 days | Beta decay to Sm-148 |
| Pm-148m | 148m | 41.29 days | Isomeric transition to Pm-148 |
| Pm-149 | 149 | 53.08 hours | Beta decay to Sm-149 |
| Pm-150 | 150 | 2.68 hours | Beta decay to Sm-150 |
| Pm-151 | 151 | 28.40 hours | Beta decay to Sm-151 |
Promethium, despite its scarcity and radioactivity, has several specialized applications due to its unique properties:
Promethium is a rare and radioactive element, not found in significant quantities in nature due to its instability. Its production is predominantly synthetic, achieved through nuclear reactions in reactors or particle accelerators. Here are the primary methods for producing promethium:
Promethium, a rare and radioactive element, finds its use in a variety of niche applications due to its unique properties. Here are some of the key applications of promethium:
Promethium, with its rare and radioactive nature, occupies a unique niche in scientific and technological applications. From powering self-luminous paint and nuclear batteries to contributing to space exploration and industrial processes, its utility extends across various domains. Despite handling challenges, the continued exploration of promethium’s potential underscores its invaluable role in advancing both technology and research.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of promethium?
60
61
62
63
Promethium is a member of which group in the periodic table?
Alkali metals
Alkaline earth metals
Lanthanides
Actinides
What is the symbol for promethium?
Pm
Pr
Po
Pt
Which of the following isotopes of promethium is the most stable?
Pm-145
Pm-146
Pm-147
Pm-148
Promethium is primarily obtained from which source?
Uranium ores
Thorium ores
Meteorites
Nuclear reactors
What type of element is promethium?
Nonmetal
Metalloid
Metal
Noble gas
Which property is not characteristic of promethium?
Radioactivity
Conductivity
Magnetism
Colorless gas
What is the oxidation state commonly exhibited by promethium in compounds?
+1
+2
+3
+4
Promethium was discovered by which method?
Spectroscopy
X-ray diffraction
Chemical synthesis
Extraction from minerals
Which application commonly uses promethium?
Smoke detectors
Watches
Beta batteries
MRI machines
Before you leave, take our quick quiz to enhance your learning!

