What is the atomic number of samarium?
62
63
64
65
Samarium, a versatile and rare earth element, holds a pivotal role in various industries, from electronics to nuclear reactors. Its unique properties, including magnetic, optical, and chemical capabilities, make it invaluable in crafting magnets, lasers, and cancer treatment drugs. As we delve into the specifics of Samarium, this guide aims to unravel its multifaceted applications and benefits. By incorporating insights into its extraction, uses, and advancements, we aim to provide a comprehensive and keyword-rich introduction that is both friendly, shedding light on this lesser-known, yet crucial, element in modern technology and medicine.
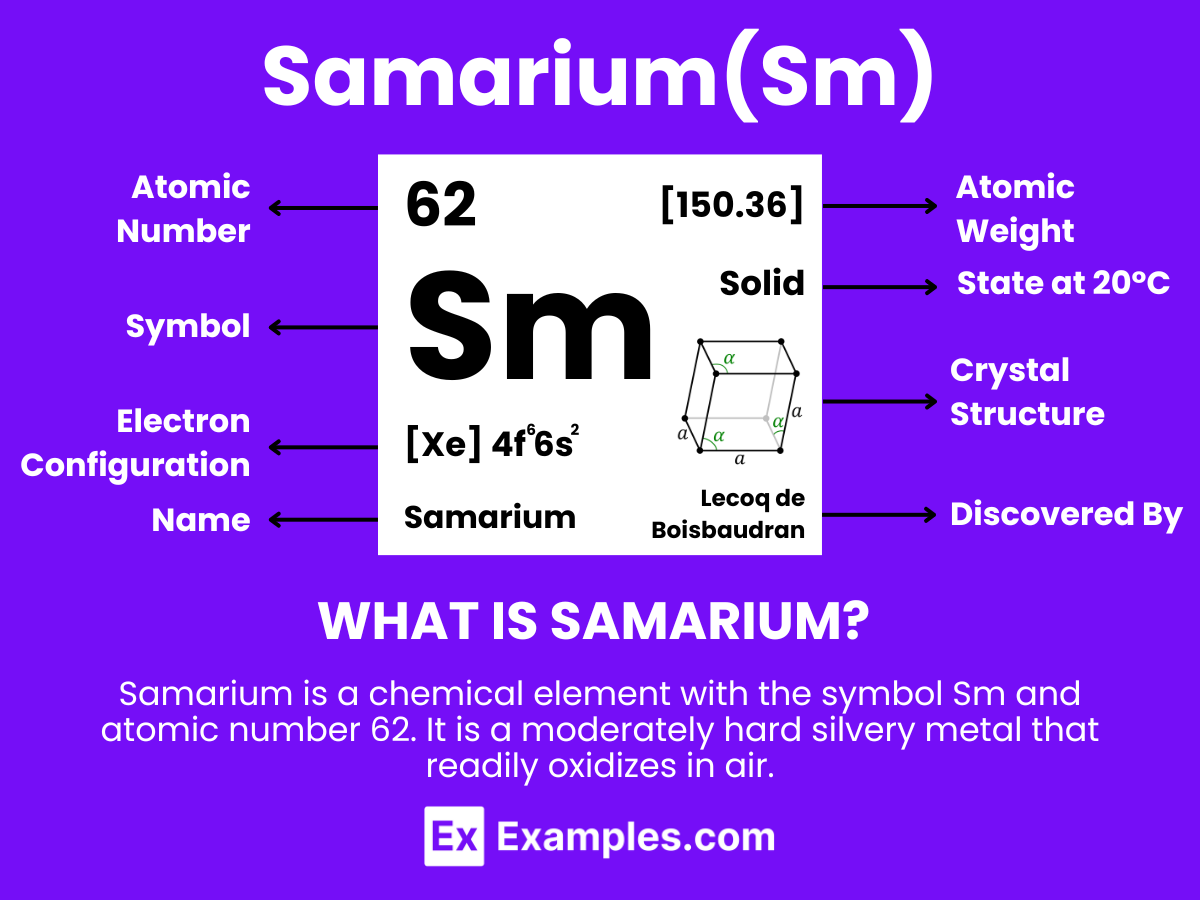
Samarium is a chemical element with the symbol Sm and atomic number 62. It is a moderately hard silvery metal that readily oxidizes in air. As a lanthanide, which is a type of rare earth element, Samarium possesses typical properties of this group, including brightness, high magnetic susceptibility, and specific electronic configurations that contribute to its unique optical and chemical behaviors. It is found in various minerals, including monazite and bastnäsite, often in association with other rare earth elements. Samarium has several applications, most notably in Samarium-Cobalt (SmCo) magnets, which are known for their high magnetic strength and thermal stability. It’s also used in cancer treatment, as a catalyst in organic chemical reactions, in glass and ceramics coloring, and as a neutron absorber in nuclear reactors.
Samarium, with the chemical symbol Sm and atomic number 62, is a fascinating element that lies within the lanthanide series of the periodic table. Its atomic structure is characterized by its unique configuration of electrons, protons, and neutrons, which gives rise to its distinctive properties and applications.
Protons and Neutrons: In the nucleus of a samarium atom, there are 62 protons, which define the element’s atomic number and its identity as samarium. The number of neutrons in samarium can vary, leading to different isotopes of the element. The most stable and naturally occurring isotope of samarium has 88 neutrons.
Electrons and Electron Configuration: Samarium has 62 electrons, with their arrangement in shells around the nucleus determined by the principles of quantum mechanics. The electron configuration of samarium is [Xe] 4f⁶ 6s², indicating that it has six electrons in the 4f subshell and two electrons in the 6s subshell, following the noble gas xenon (Xe) in its electron configuration. This configuration is crucial for understanding the chemical behavior and reactivity of samarium, particularly its valence and the types of chemical bonds it can form.
Atomic Structure and Properties: The atomic structure of samarium contributes to its metallic nature, including its lustrous appearance and good electrical conductivity. The arrangement of electrons in the 4f subshell is partially responsible for the magnetic properties of samarium, especially its use in making samarium-cobalt magnets, which are known for their high magnetic strength and resistance to demagnetization.
Role in the Periodic Table: Positioned among the lanthanides, samarium exhibits typical properties of rare earth elements, such as high melting and boiling points, and the ability to form trivalent ions (Sm³⁺). These ions are key to samarium’s behavior in various compounds and its applications in materials science, technology, and even medicine.
Samarium, a lanthanide element with the symbol Sm and atomic number 62, exhibits several chemical properties that are typical of rare earth metals. Below, the chemical properties of samarium are described in detail, along with relevant equations to illustrate its reactivity and formation of compounds.
| Property | Value | Units |
|---|---|---|
| Melting Point | 1345 K | Kelvin |
| Boiling Point | 2076 K | Kelvin |
| Heat of Fusion | 8.62 kJ/mol | Kilojoules per mole |
| Heat of Vaporization | 192 kJ/mol | Kilojoules per mole |
| Specific Heat Capacity (at 25°C) | 29.54 J/mol·K | Joules per mole Kelvin |
| Thermal Conductivity | 13.3 W/(m·K) | Watts per meter Kelvin |
| Property | Value | Units |
|---|---|---|
| Density | 7.52 g/cm³ | Grams per cubic centimeter |
| Mohs Hardness | ~5 | Scale |
| Young’s Modulus | 49.7 GPa | Gigapascals |
| Poisson’s Ratio | 0.274 | Dimensionless |
| Brinell Hardness | Approximately 500 MPa | Megapascals |
| Crystal Structure | Rhombohedral | – |
| Property | Value | Units |
|---|---|---|
| Electrical Resistivity | 0.940 µΩ·m | Microohm meters |
| Magnetic Ordering | Paramagnetic at 300 K | – |
| Curie Temperature | Not applicable (N/A) | Kelvin |
| Magnetic Moment | 0.71 µB/Samarium atom | Bohr magnetons |
| Property | Value | Units |
|---|---|---|
| Natural Isotopes | ¹⁴⁴Sm, ¹⁴⁹Sm, ¹⁵⁰Sm, ¹⁵²Sm, ¹⁵⁴Sm | – |
| Most Stable Isotope | ¹⁵²Sm (Stable) | – |
| Neutron Cross Section (¹⁴⁹Sm) | 41,000 barns | Barns |
| Isotopic Abundance (Natural) | ¹⁴⁴Sm: 3.07%, ¹⁴⁹Sm: 13.82%, ¹⁵²Sm: 26.75%, ¹⁵⁴Sm: 22.75% | Percent |
Samarium, a rare earth element with significant industrial and technological applications, is obtained through a series of complex processes from its ores, primarily from monazite and bastnasite. These ores contain a mixture of different lanthanides, including samarium, which necessitates a detailed separation process to isolate pure samarium. The preparation of samarium involves several key steps:
Extraction from Ores: The initial step in the preparation of samarium is the extraction of the element from its ore. This is typically done using acid leaching, where the ore is treated with a strong acid, such as hydrochloric acid or sulfuric acid, to dissolve the rare earth elements into a solution.
Ion Exchange and Solvent Extraction: Once the rare earth elements are in solution, samarium is separated from the other lanthanides using ion exchange or solvent extraction techniques. These methods exploit the slight differences in chemical properties among the lanthanides to selectively bind or dissolve certain elements while leaving others behind. Solvent extraction, for example, involves the use of organic solvents that selectively react with samarium ions, allowing for their separation from the mixture.
Precipitation and Calcination: After separation, the samarium is often precipitated out of the solution as samarium oxalate or carbonate. This precipitate is then filtered, washed, and dried. Following precipitation, the samarium compound is subjected to calcination – a process of heating to a high temperature in the absence of air or in a controlled atmosphere. This step converts the samarium compound into samarium oxide (Sm2O3), a common form of the element that is easier to handle and process further.
Metallic Samarium Production: To obtain metallic samarium, the samarium oxide is mixed with a reducing agent, such as lanthanum metal or calcium, and heated in a vacuum or inert atmosphere. This reduction process removes the oxygen atoms from the samarium oxide, leaving behind pure samarium metal. The reaction typically occurs at high temperatures, and the resulting samarium metal can then be cast into ingots, powders, or other desired forms.
Refining: The produced samarium metal may undergo further refining processes to increase its purity. Electrorefining and vacuum distillation are common methods used to refine samarium, depending on the required level of purity and the intended application of the metal.
1.Samarium Oxide (Sm₂O₃)
2.Samarium Chloride (SmCl₃)
3.Samarium Fluoride (SmF₃)
4.Samarium Boride (SmB₆)
5.Samarium Cobalt (SmCo₅)
6.Samarium Sulfide (SmS)
| Isotope | Mass Number | Half-Life | Key Characteristics |
|---|---|---|---|
| Sm-144 | 144 | Stable | Non-radioactive; one of the naturally occurring stable isotopes of samarium. |
| Sm-147 | 147 | 1.06 × 10¹¹ years | Used for dating geological materials; alpha emitter. |
| Sm-148 | 148 | Stable | Non-radioactive; contributes to the natural occurrence of samarium. |
| Sm-149 | 149 | Stable | Absorbs neutrons; used in nuclear reactors. |
| Sm-150 | 150 | Stable | Non-radioactive; another stable isotope contributing to samarium’s natural presence. |
| Sm-152 | 152 | Stable | Has a high neutron absorption cross-section; used in nuclear technology. |
| Sm-153 | 153 | 46.3 hours | Used in medicine for treating pain in cancerous bones. |
| Sm-154 | 154 | Stable | Non-radioactive; the heaviest naturally occurring stable isotope of samarium. |
Samarium, a rare earth metal with unique chemical and physical properties, plays a crucial role in various advanced technologies and industries. Its diverse applications stem from its magnetic, optical, and chemical characteristics, making it an essential element in modern-day devices and solutions. Here’s a look at some of the key applications of samarium:
Permanent Magnets: Samarium is perhaps best known for its use in Samarium-Cobalt (SmCo) magnets, which are among the strongest types of permanent magnets. These magnets exhibit excellent thermal stability and resistance to demagnetization, making them ideal for applications in aerospace, military, and high-performance motors where reliability and durability are critical.
Cancer Treatment: Samarium-153 is utilized in the medical field as a part of a compound called samarium-153 lexidronam, which is used in the treatment of pain associated with cancerous bone tumors. This radioisotope helps relieve pain by delivering targeted radiation therapy to the affected bones.
Nuclear Reactors: Certain isotopes of samarium, such as Samarium-149, have a high neutron absorption capacity, making them valuable as control materials in nuclear reactors. They help regulate the reactor’s power output by absorbing excess neutrons, thus contributing to the safety and efficiency of nuclear power generation.
Catalysts: Samarium oxide (Sm₂O₃) serves as a catalyst in several chemical reactions, including the dehydration and dehydrogenation of ethanol. Its catalytic properties are also explored in organic synthesis and the production of fine chemicals.
Optical and Infrared Applications: Samarium’s optical properties make it useful in the manufacturing of special glasses and ceramics that require specific light absorption characteristics. For example, samarium-doped glasses can absorb infrared light, making them suitable for various optical applications, such as protecting eyewear from laser beams.
Electronics and Telecommunications: Samarium compounds, like samarium oxide, are used in electronic and telecommunication equipment for their dielectric properties. They contribute to the miniaturization and enhancement of capacitors and other components critical to modern electronic devices.
Quantum Computing and Research: The unique properties of samarium and its compounds are subjects of interest in quantum computing and advanced research areas. Studies explore the potential of samarium-based materials in developing new quantum computing architectures and memory storage technologies.
This article has comprehensively outlined the multifaceted aspects of Samarium, including its thermodynamic, material, electromagnetic, and nuclear properties, along with its chemical compounds, isotopes, diverse applications, and detailed production process. Samarium’s unique characteristics make it invaluable across numerous fields, from technology and healthcare to energy and manufacturing, highlighting its significance in advancing modern innovations.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of samarium?
62
63
64
65
Samarium belongs to which group of elements in the periodic table?
Transition metals
Alkali metals
Lanthanides
Actinides
What is the chemical symbol for samarium?
Sm
Sa
Sr
Sc
Samarium is primarily used in which application?
Catalysts
Magnets
Batteries
Semiconductors
What is the most common oxidation state of samarium in its compounds?
+1
+2
+3
+4
Which of the following is a property of samarium?
High electrical conductivity
Magnetic properties
Low density
Non-metallic nature
Samarium is extracted from which type of minerals?
Bauxite and hematite
Monazite and bastnasite
Sphalerite and galena
Rutile and ilmenite
What is the melting point of samarium?
631°C
1072°C
1345°C
1545°C
Which isotope of samarium is used in the production of nuclear reactor control rods?
Samarium-144
Samarium-147
Samarium-149
Samarium-152
Samarium forms an alloy with cobalt known as:
SmCo
SmCu
SmFe
SmNi
Before you leave, take our quick quiz to enhance your learning!

