Seaborgium (Sg) – Definition, Preparation, Properties, Uses, Compounds, Reactivity
Dive into the world of Seaborgium, a synthetic marvel in the periodic table, with this complete guide. Seaborgium, a superheavy element known for its elusive nature and intriguing properties, captivates scientists and enthusiasts alike. In this guide, we’ll explore the definition, meaning, and the potential uses of Seaborgium, alongside a closer look at its compounds. Through engaging examples, discover the significance of Seaborgium in advancing our understanding of chemistry and its role in scientific research, offering a unique perspective on this fascinating element.
What is Seaborgium?
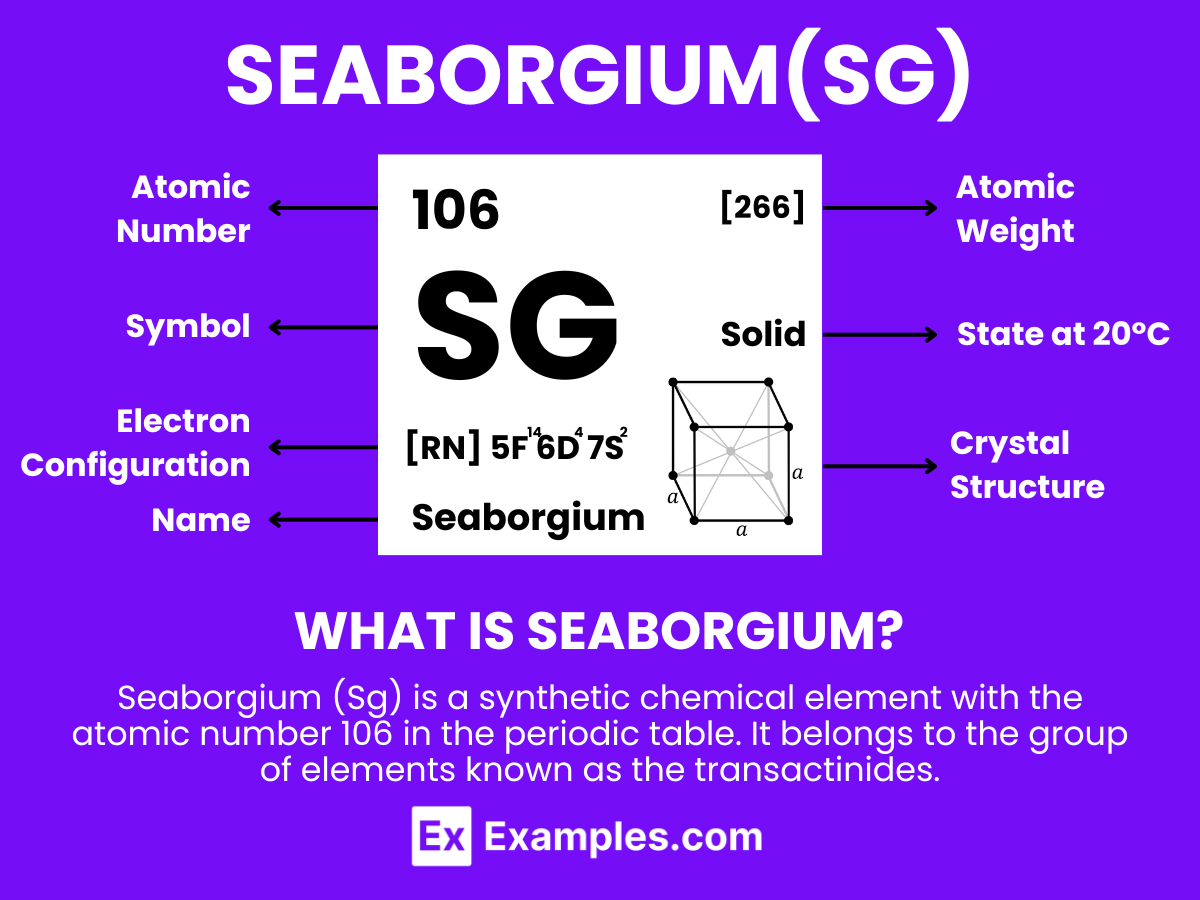
Seaborgium (Sg) is a synthetic chemical element with the atomic number 106 in the periodic table. It belongs to the group of elements known as the transactinides. Seaborgium was first synthesized in 1974 through a collaborative effort by scientists at the Lawrence Berkeley National Laboratory in the United States and the Joint Institute for Nuclear Research in the Soviet Union. The element was named in honor of Glenn T. Seaborg, a Nobel Prize-winning chemist who was instrumental in the discovery of several transuranium elements.
Seaborgium Formula
- Formula: Sg
- Composition: Composed of a single seaborgium atom.
- Bond Type: In its elemental form, seaborgium does not engage in bonding as it exists as a pure element. However, it has the potential to form both covalent and ionic bonds when interacting with other elements, although its chemical behavior is largely unexplored due to its transient nature and scarcity.
- Molecular Structure: Seaborgium, as an individual element, does not form traditional molecular structures. It is theorized to display the characteristics of a heavy metal, possibly with a close-packed crystalline structure, though these assumptions are speculative.
- Electron Sharing: Seaborgium is anticipated to participate in electron sharing through covalent bonds or electron transfer in ionic bonds with different elements. These predictions are made based on its position within the periodic table, yet direct experimental evidence is limited.
- Significance: As a superheavy element created in particle accelerators, seaborgium provides valuable insights into the properties and behaviors of elements at the extreme end of the periodic table, contributing to our understanding of heavy nuclei stability.
- Role in Chemistry: Seaborgium’s primary role is within scientific exploration, specifically in research focused on expanding the known boundaries of the periodic table and synthesizing new elements. Practical applications of seaborgium are currently theoretical, with its significance tied to advancing nuclear chemistry and physics.
Atomic Structure of Seaborgium
Seaborgium (Sg) is a synthetic element with the atomic number 106, positioned in the transactinide series of the periodic table. It belongs to the d-block, specifically Group 6, sharing this category with elements like chromium, molybdenum, tungsten, and seaborgium’s immediate predecessor, dubnium. Due to its position, it’s presumed to display characteristics and behaviors akin to those of its Group 6 counterparts, albeit modified by its significantly higher atomic number.
Electron Configuration
The electron configuration of seaborgium is theorized to be [Rn] 5f¹⁴ 6d⁴ 7s², indicating that it has six electrons in its outermost shell. This configuration suggests seaborgium’s potential to form various oxidation states, with +6 being the most stable and common, similar to the lighter members of its group.
Bond Type and Molecular Structure
In its elemental form, seaborgium does not form bonds as it exists as a single atom. However, it is capable of forming covalent or ionic bonds when interacting with other elements. The molecular structure of seaborgium compounds would be determined by the type of bonds it forms, though direct observations are limited due to the element’s short half-life and the challenge of producing it in significant amounts.
Electron Sharing
Seaborgium is expected to participate in electron sharing through covalent bonding or electron transfer in ionic interactions. Predictions about its chemical behavior are primarily based on theoretical models and the properties of its lighter group members, as empirical data on seaborgium is scarce.
Significance and Role in Chemistry
The study of seaborgium’s atomic structure provides valuable insights into the chemical and physical properties of superheavy elements. As a part of ongoing research in nuclear chemistry, seaborgium helps scientists explore the limits of the periodic table, the stability of heavy nuclei, and the theoretical predictions of quantum mechanics on elemental behavior.
Properties of Seaborgium
Physical Properties of Seaborgium
| Physical Property | Description |
|---|---|
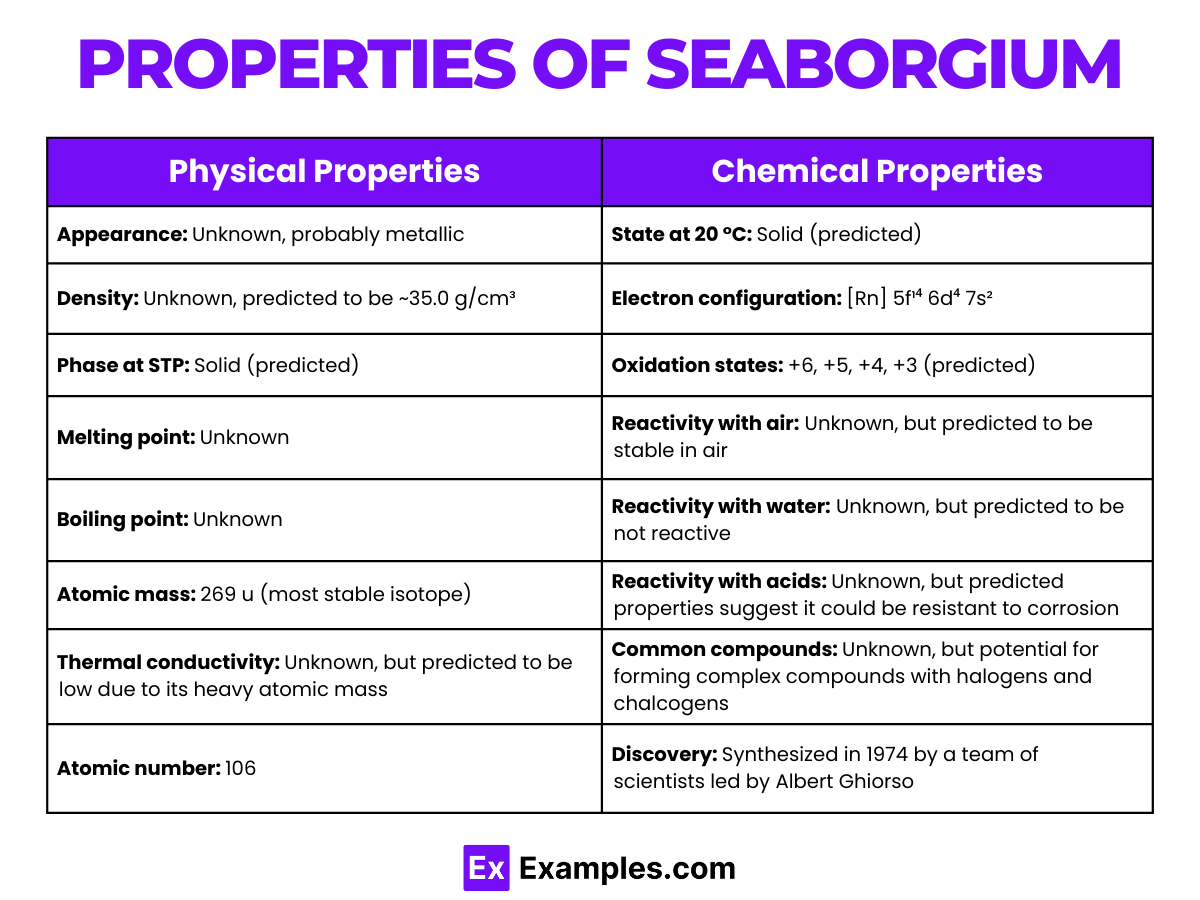
| Atomic Number | 106 |
| Atomic Mass | [269] (most stable isotope) |
| State at Room Temperature | Presumed to be solid |
| Melting Point | Unknown; predicted based on group trends |
| Boiling Point | Unknown; predicted based on group trends |
| Density | Theoretical; expected to be high |
| Color | Unknown; presumed to be metallic |
| Radioactivity | Highly radioactive with a short half-life |
Chemical Properties of Seaborgium
Seaborgium (Sg), with atomic number 106, is a superheavy synthetic element belonging to Group 6 of the periodic table, alongside elements such as chromium, molybdenum, and tungsten. Its chemical properties have been explored to a limited extent due to its short half-life and the technical challenges in producing it. However, theoretical studies and indirect experimental data provide insights into its potential chemical behavior, primarily based on its position in the periodic table and its relationship to lighter homologs. Here we detail the anticipated chemical properties of Seaborgium, supported by relevant theoretical equations where possible.
Oxidation States
Seaborgium is expected to exhibit various oxidation states, with +6 being the most stable and common, mirroring the chemistry of other Group 6 elements. This predicts its ability to form compounds analogous to those of tungsten and molybdenum.
Formation of Halides
Seaborgium is predicted to form halides, particularly in the +6 oxidation state, suggesting compounds such as SgF₆. An example theoretical reaction could be:
This equation suggests the formation of seaborgium hexafluoride, a compound analogous to WF₆ and MoF₆.
Oxides
Similarly, seaborgium is expected to form stable oxides in its highest oxidation state. A predicted oxide formation might involve:
indicating the formation of seaborgium trioxide, SgO₃, under oxidizing conditions.
Interaction with Organic Ligands
Given its expected chemical similarities to other Group 6 elements, seaborgium may also form complex compounds with organic ligands, potentially useful in studying its chemical behavior in solution. However, specific reactions and compounds remain speculative.
Aqueous Chemistry
In aqueous solutions, seaborgium is anticipated to behave similarly to its lighter homologs, forming complex ions such as SgO₂⁺⁴ in strong oxidizing conditions, indicative of its +6 oxidation state.
Stability and Reactivity
The chemical stability and reactivity of seaborgium compounds are expected to be influenced by relativistic effects, due to the element’s high atomic number. These effects may cause seaborgium’s chemical properties to deviate somewhat from those predicted by simple extrapolation from lighter elements.
Significance in Research
Although direct experimental verification of seaborgium’s chemical properties is limited, the study of its compounds and reactions contributes to the understanding of the chemistry of superheavy elements. It offers insights into the effects of relativistic changes on chemical behavior and helps refine theoretical models of atomic and molecular structures.
Nuclear Properties of Seaborgium
| Property | Description |
|---|---|
| Half-lives | Varies by isotope; generally short-lived |
| Isotopes | Known isotopes range from Sg-258 to Sg-271 |
| Nuclear Spin | Theoretical prediction for specific isotopes |
| Decay Modes | Primarily alpha decay; some isotopes may undergo spontaneous fission |
Preparation of Seaborgium
The preparation of Seaborgium (Sg), element 106, is a complex process that requires a particle accelerator, sophisticated detection equipment, and a deep understanding of nuclear physics. Seaborgium does not occur naturally and is produced in laboratory settings by bombarding target atoms of lighter elements with high-energy beams of ions. The most common method involves heavy ion reactions, where a lighter element is accelerated and directed to collide with a heavier target element, leading to the fusion of their nuclei and the formation of Seaborgium.
Typical Production Process:
- Selection of Target and Projectile: A heavy element, such as lead (Pb) or bismuth (Bi), is often used as the target, with a lighter element like chromium (Cr) or iron (Fe) serving as the projectile. The choice depends on the desired isotope of Seaborgium to be synthesized.
- Acceleration: The projectile ions are accelerated to high energies using a particle accelerator, which is necessary to overcome the electrostatic repulsion between the target and the projectile nuclei.
- Collision and Fusion: The accelerated ions collide with the target material. Upon impact, the nuclei of the projectile and target atoms can fuse, forming a heavier, compound nucleus. This process typically produces a nucleus in an excited state.
- Nuclear Reaction: To form Seaborgium, the compound nucleus must lose some of its excitation energy and possibly eject one or more neutrons.
- Detection: The newly formed Seaborgium atom is then detected and identified through its decay products. Due to its highly radioactive nature, Seaborgium exists only for a short duration before undergoing decay, making immediate detection crucial.
Chemical Compounds of Seaborgium
1.Seaborgium Oxide (SgO₂)
- Equation: Sg+O₂→SgO₂
- Seaborgium oxide, indicating its reactivity towards oxygen.
2.Seaborgium Chloride (SgCl₆)
- Equation: Sg+3Cl₂→SgCl₆
- Formation of Seaborgium hexachloride, demonstrating halogen-like behavior.
3.Seaborgium Fluoride (SgF₆)
- Equation: Sg+3F₂→SgF₆
- Production of Seaborgium hexafluoride, showcasing its reactivity with fluorine.
4.Seaborgium Hydroxide (Sg(OH)₆)
- Equation:Sg⁶⁺+6OH⁻→ Sg(OH)₆
- Formation of Seaborgium hydroxide, highlighting its coordination chemistry.
5.Seaborgium Nitrate (Sg(NO₃)₄)
- Equation: Sg+4HNO₃→Sg(NO₃)₄+4H⁺+4NO₂
- Interaction with nitric acid to produce Seaborgium nitrate, indicating its behavior towards acids.
6.Seaborgium Sulfide (SgS₂)
- Equation: Sg+2S→SgS₂
- Formation of Seaborgium disulfide, demonstrating its ability to react with sulfur.
Isotopes of Seaborgium
The table below summarizes the known isotopes of Seaborgium (Sg), providing details on their mass numbers, half-lives, and primary modes of decay. As a synthetic and highly unstable element, Seaborgium’s isotopes are produced in particle accelerators and exist only momentarily before decaying into lighter elements.
| Isotope | Mass Number | Half-life | Primary Mode of Decay |
|---|---|---|---|
| Sg-258 | 258 | 2.9 milliseconds | Alpha decay |
| Sg-259 | 259 | 0.48 seconds | Alpha decay |
| Sg-260 | 260 | 3.8 milliseconds | Alpha decay |
| Sg-261 | 261 | 230 milliseconds | Alpha decay |
| Sg-262 | 262 | 6.9 milliseconds | Alpha decay |
| Sg-263 | 263 | 1 second | Alpha decay |
| Sg-264 | 264 | 37 seconds | Alpha decay |
| Sg-265 | 265 | 16.2 seconds | Alpha decay |
| Sg-266 | 266 | 21 seconds | Alpha decay, Spontaneous fission |
| Sg-267 | 267 | 1.4 minutes | Alpha decay |
| Sg-268 | 268 | 35 hours | Alpha decay |
| Sg-269 | 269 | 3.1 minutes | Alpha decay |
| Sg-270 | 270 | 10 minutes | Alpha decay, Spontaneous fission |
| Sg-271 | 271 | 2.4 minutes | Alpha decay |
Uses of Seaborgium
- Fundamental Scientific Research: Seaborgium’s synthesis and investigation provide insights into the properties of superheavy elements, enriching the scientific community’s understanding of the periodic table’s outer limits.
- Nuclear Stability Studies: Research involving Seaborgium contributes to the theoretical and experimental understanding of nuclear stability, particularly in the context of the “island of stability,” a concept suggesting a region in the periodic table where superheavy elements might have longer half-lives.
- Chemical Property Exploration: Though direct chemical experimentation with Seaborgium is limited, theoretical studies and comparison with lighter homologs help predict its chemical properties, contributing to the broader field of chemistry, especially in understanding element behavior in extreme conditions.
- Particle Physics Advancements: The production of Seaborgium advances particle accelerator technology and nuclear physics techniques, contributing to developments that have wider applications in science, from materials research to medical isotope production.
- Educational and Inspirational Value: The quest to discover and study Seaborgium serves as an educational tool, illustrating the complexities of element synthesis and the scientific method, thereby inspiring future generations of scientists and enriching science education.
- Technological Development: The challenges associated with Seaborgium’s detection and analysis drive innovation in instrumentation and experimental methods, which can be applied in other areas of physics and chemistry, enhancing analytical capabilities across scientific disciplines.
Production of Seaborgium
The production of Seaborgium (Sg) is a sophisticated process that occurs within particle accelerators, requiring the collision of lighter nuclei at high energies to create this superheavy element.
- Target Selection: A target material, often made of a heavy element like lead or bismuth, is prepared. This material will receive the ion beam generated by the accelerator.
- Ion Acceleration: Ions of a lighter element, such as chromium or iron, are accelerated to high velocities within a particle accelerator. These ions must reach sufficient energy to overcome the electrostatic repulsion of the target nuclei.
- Nuclear Reaction: The accelerated ions collide with the target atoms. Under the right conditions, a fusion reaction occurs, producing an atom of Seaborgium and typically one or more neutrons.
- Detection and Identification: The newly formed Seaborgium atom is extremely unstable and decays rapidly. Its presence is confirmed through the detection of decay products, using sophisticated instrumentation designed to identify the specific signatures of superheavy elements.
Applications of Seaborgium
Seaborgium’s applications are primarily academic, given its extremely short half-life and the challenges associated with its production. Despite these limitations, the study of Seaborgium has several important applications:
- Theoretical Chemistry and Physics: Understanding Seaborgium’s properties helps refine theoretical models of atomic structure and bonding, contributing to the broader knowledge base of chemistry and physics.
- Nuclear Stability Research: Seaborgium plays a crucial role in the study of the stability of superheavy elements, aiding in the search for the predicted “island of stability” where elements might have longer half-lives.
- Periodic Table Expansion: The synthesis of Seaborgium and its characterization contribute to the expansion and refinement of the periodic table, particularly in understanding the properties and behaviors of elements in its region.
- Advancements in Particle Acceleration and Detection Technology: The techniques developed for Seaborgium’s production and detection push the boundaries of technology, with potential applications in various scientific fields, including materials science and medical diagnostics.
- Interdisciplinary Collaboration: The efforts to produce and study Seaborgium encourage collaboration among scientists from diverse fields, fostering advances in nuclear chemistry, physics, and beyond.
- Educational Resource: The quest to discover new elements and understand their properties provides a compelling narrative for educational purposes, inspiring future scientists and enriching science curricula across disciplines.
Seaborgium stands at the frontier of scientific exploration, embodying the relentless pursuit of knowledge that defines the field of nuclear chemistry. Though its practical applications are limited by its fleeting existence, the synthesis and study of Seaborgium illuminate the complexities of superheavy elements, pushing the boundaries of the periodic table and enhancing our understanding of the atomic world.