What is the atomic number of Thorium?
88
90
91
92
Discover the fascinating world of Thorium, a remarkable element with significant potential in various fields, including energy production and material science. This comprehensive guide delves into Thorium’s properties, uses, and the innovative compounds it forms, providing insightful examples that illustrate its versatility and importance. Whether for nuclear reactors or cutting-edge research, Thorium stands out as a key player in advancing technology and sustainability.
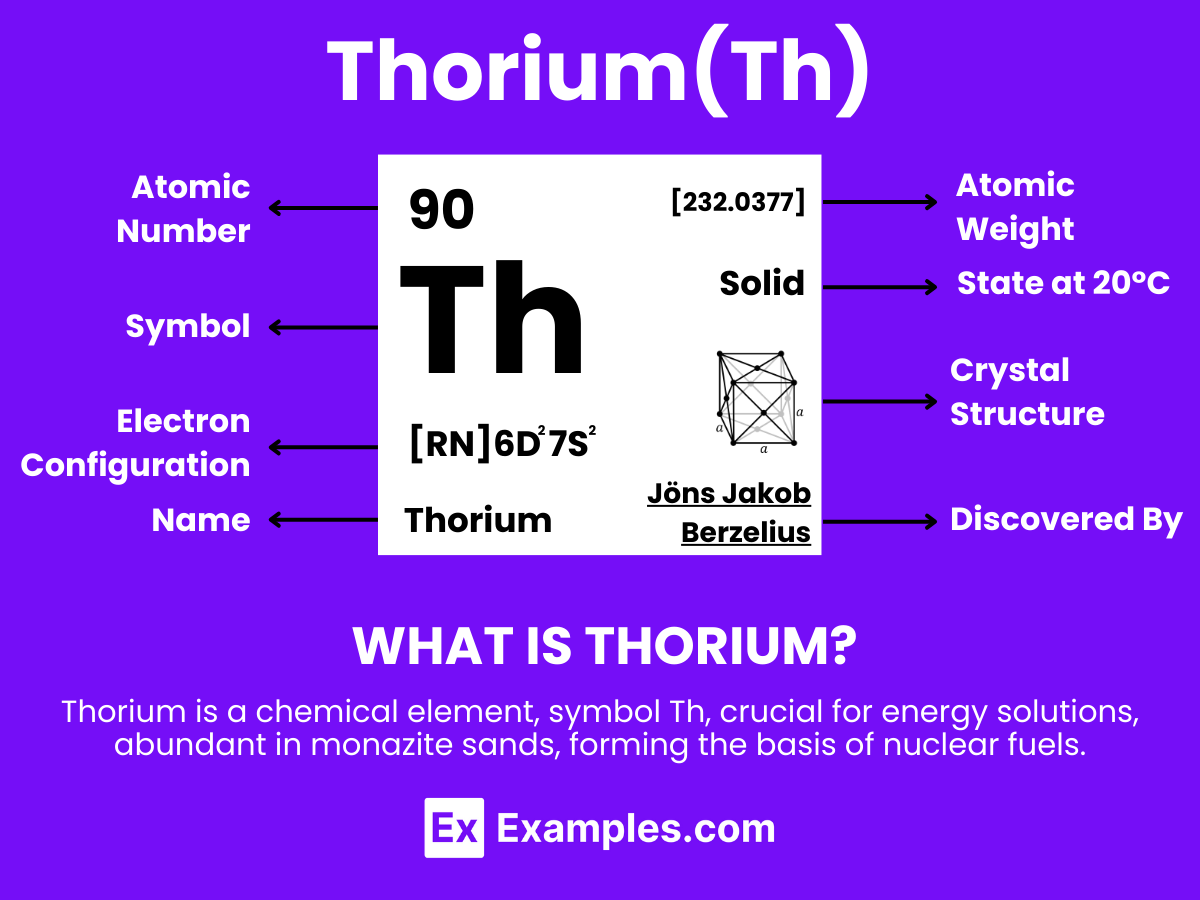
Thorium is a dense, silvery metal that stands out due to its significant properties and diverse applications. With the atomic number 90, Thorium is recognized for its remarkable capacity for energy production, especially as a potential fuel in nuclear reactors due to its abundance and safety advantages over traditional uranium-based fuels. This element is naturally occurring and can be found in small amounts in rocks and soils worldwide, often associated with rare earth minerals from which it is extracted. Thorium’s use extends beyond energy production; it also finds applications in materials science for high-temperature ceramics, as well as in the production of gas mantles and as a radiographic contrast agent in the medical field. Its potential to serve as a cleaner, safer nuclear fuel highlights its importance in future energy strategies, driving research and development efforts to harness its full capabilities
The atomic structure of thorium, a naturally occurring radioactive element, plays a crucial role in its physical and chemical properties. Thorium is represented by the symbol Th and has the atomic number 90. Here’s a detailed look into the atomic structure of thorium:
The electron configuration of thorium is [Rn] 6d² 7s², indicating that it has two electrons in the 6d orbital and two electrons in the 7s orbital, beyond the filled orbitals of radon (Rn). This configuration is crucial for understanding thorium’s chemical behavior, particularly its valence and bonding characteristics.
The nucleus of a thorium atom contains 90 protons, which positively charge the nucleus, and a varying number of neutrons among its isotopes, with ^232Th having 142 neutrons. The balance between protons and neutrons is vital for the stability of the nucleus, influencing the radioactive decay processes thorium undergoes.
Thorium’s atomic structure is not only fascinating from a scientific standpoint but also underpins its potential in nuclear energy applications. Thorium-232 can absorb neutrons to become thorium-233, which decays into protactinium-233 and then into uranium-233, a fissile material. This pathway is the basis for thorium’s role in proposed future nuclear reactors, which aim to be safer and produce less long-lived radioactive waste compared to current uranium-based reactors
| Property | Value |
|---|---|
| Appearance | Silvery, often with black tarnish |
| Atomic Number | 90 |
| Atomic Weight | 232.03806 u |
| Density | 11.7 g/cm³ (at 20 °C) |
| Melting Point | 1750 °C (3182 °F; 2023 K) |
| Boiling Point | 4788 °C (8650 °F; 5061 K) |
| State at 20 °C | Solid |
| Crystal Structure | Face-centered cubic (fcc) |
| Thermal Conductivity | 54 W/(m·K) (at 300 K) |
| Electrical Resistivity | ~15 nΩ·m (at room temperature) |
| Thermal Expansion | 11.0 µm/(m·K) (at 25 °C) |
| Young’s Modulus | 79 GPa |
| Shear Modulus | 31 GPa |
| Bulk Modulus | 54 GPa |
| Mohs Hardness | 3 |
| CAS Number | 7440-29-1 |
Thorium is a naturally occurring, radioactive chemical element with the symbol Th and atomic number 90. It is part of the actinide series in the periodic table. Being a radioactive element, its most stable isotope, Thorium-232, has a half-life of approximately 14.05 billion years, which is roughly the age of the universe. Thorium’s properties, including its chemical behavior, are influenced by its electron configuration and its position in the periodic table.
Thorium primarily exhibits a +4 oxidation state in its compounds, which is the most stable state due to the full filling of the 6d and 5f orbitals. However, lower oxidation states have been observed in some compounds, though they are less stable.
Thorium metal is fairly reactive. When exposed to air, it tarnishes and forms thorium dioxide (ThO₂): Th+2O₂→ThO₂
Thorium reacts slowly with water, forming thorium dioxide and releasing hydrogen gas: Th+2H₂O→ThO₂+2H₂
Thorium readily dissolves in hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3), forming thorium(IV) salts and releasing hydrogen gas:
Th+4HCl→ThCl4+2H₂
Th+H₂SO₄→ThSO₄+H₂
Th+4HNO₃ →Th(NO₃ )₄+2H₂
Thorium does not react with most alkalis at room temperature, but it can form thorates with powerful oxidizing agents or at high temperatures.
While thorium’s chemical properties are significant, its radioactivity cannot be overlooked. Thorium-232 decays via alpha emission to radium-228, as part of the thorium decay series: 232Th→228Ra+α
Thorium compounds, such as thorium nitrate, are soluble in water and organic solvents. However, thorium dioxide is insoluble in water but can dissolve in concentrated acids.
| Property | Value | Conditions | Units |
|---|---|---|---|
| Melting Point | 2023 | K (Kelvin) | |
| Boiling Point | 5061 | K (Kelvin) | |
| Heat of Fusion | 16.11 | kJ/mol | |
| Heat of Vaporization | 514.4 | kJ/mol | |
| Specific Heat Capacity | 26.230 | at 25°C (298 K) | J/(mol·K) |
| Thermal Conductivity | 54 | at 300 K | W/(m·K) |
| Thermal Expansion | 11.0 | at 25°C (298 K) | µm/(m·K) |
| Property | Value | Conditions | Units |
|---|---|---|---|
| Density | 11.7 | at 20°C | g/cm³ |
| Mohs Hardness | ~3 | ||
| Young’s Modulus | 79 | GPa | |
| Poisson’s Ratio | 0.27 | ||
| Brinell Hardness | 400 | MPa | |
| Vickers Hardness | 295-685 | MPa |
| Property | Value | Conditions | Units |
|---|---|---|---|
| Electrical Resistivity | 157 | at 20°C | nΩ·m |
| Magnetic Ordering | Paramagnetic | ||
| Superconducting Point | <2.1 | K (Kelvin) |
| Property | Value | Conditions | Units |
|---|---|---|---|
| Atomic Number (Z) | 90 | ||
| Atomic Mass (A) | 232.03806 | for Thorium-232 | u (Unified Atomic Mass Units) |
| Half-Life of ^232Th | 14.05 billion years | Years | |
| Major Isotopes | ^232Th | Most abundant isotope | |
| Neutron Cross Section | 7.4 | Thermal neutron capture | barns |
| Neutron Mass Absorption | 0.00004 |
Thorium (Th) is a naturally occurring radioactive element, primarily found in the mineral monazite. The preparation of thorium from its ores involves a series of steps to extract and purify the element. Here’s a simplified overview of the process:
| Isotope | Mass Number | Half-Life | Decay Mode |
|---|---|---|---|
| Th-230 | 230 | 75,380 years | Alpha decay to Ra-226 |
| Th-231 | 231 | 25.52 hours | Beta decay to Pa-231 |
| Th-232 | 232 | 14.05 billion years | Alpha decay to Ra-228 |
| Th-233 | 233 | 22.3 minutes | Beta decay to Pa-233 |
| Th-234 | 234 | 24.10 days | Beta decay to Pa-234 |
Thorium-232, the most abundant and stable isotope, serves as the primary focus in thorium research and applications, particularly in nuclear technology.
The production of thorium primarily involves the processing of various thorium-containing minerals and ores. The most common source of thorium is monazite, a phosphate mineral that contains between 0.2% to 2.0% thorium oxide (ThO2) along with rare earth elements. Below are the key steps and methods used in the production of thorium:
Thorium finds application in several fields, leveraging its unique nuclear properties and relative abundance. Some of the key applications of thorium include:
Thorium presents a fascinating blend of physical and chemical properties, offering significant potential for energy and technological applications. Its preparation from monazite ore through a series of extraction and purification steps yields pure thorium, which can be utilized in various forms. The exploration of thorium’s capabilities continues to advance, promising innovative solutions for future challenges.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Thorium?
88
90
91
92
What is the chemical symbol for Thorium?
Th
To
Tr
Tm
In which block of the periodic table is Thorium found?
s-block
p-block
d-block
f-block
Which of the following is a common isotope of Thorium?
Th-230
Th-231
Th-232
Th-233
What is the half-life of Thorium-232?
14 billion years
5,000 years
75,000 years
1.4 billion years
Thorium is primarily used in which industry?
Electronics
Agriculture
Nuclear energy
Textile
What is the primary ore from which Thorium is extracted?
Bauxite
Hematite
Monazite
Galena
Which property of Thorium makes it useful as a nuclear fuel?
High melting point
High neutron absorption cross-section
Low toxicity
High electrical conductivity
Which country holds the largest reserves of Thorium?
Australia
Canada
India
Russia
What is the primary advantage of using Thorium over Uranium in nuclear reactors?
Higher energy density
Lower radioactivity
More abundant
Easier to mine
Before you leave, take our quick quiz to enhance your learning!

