What is the primary use of phosphoric acid in the methanol industry?
Catalyst
Solvent
Reactant
Stabilizer


Methanol, often referred to in chemistry as a simple yet fascinating molecular compound, is like a building block in the vast world of science. Think of it as a kind of alcohol, but not the kind you find in beverages. Instead, methanol is used in making various products, from plastics to paints, and even as fuel for some racing cars. Its structure is quite straightforward, composed of one carbon atom connected to three hydrogen atoms and one oxygen atom, which is also bonded to a hydrogen atom. This neat arrangement makes methanol a key player in many chemical reactions, showcasing the incredible versatility and importance of molecular compounds in our daily lives and the broader field of chemistry.
| Property | Value |
|---|---|
| Formula | CH₃OH |
| Hill formula | CH₄O |
| Name | Methanol |
| Alternate Names | Carbinol, Colonial spirit, Methyl alcohol, Methyl hydroxide, Methylol, Pyroxylic spirit, Wood alcohol, Wood naphtha, Wood spirit |
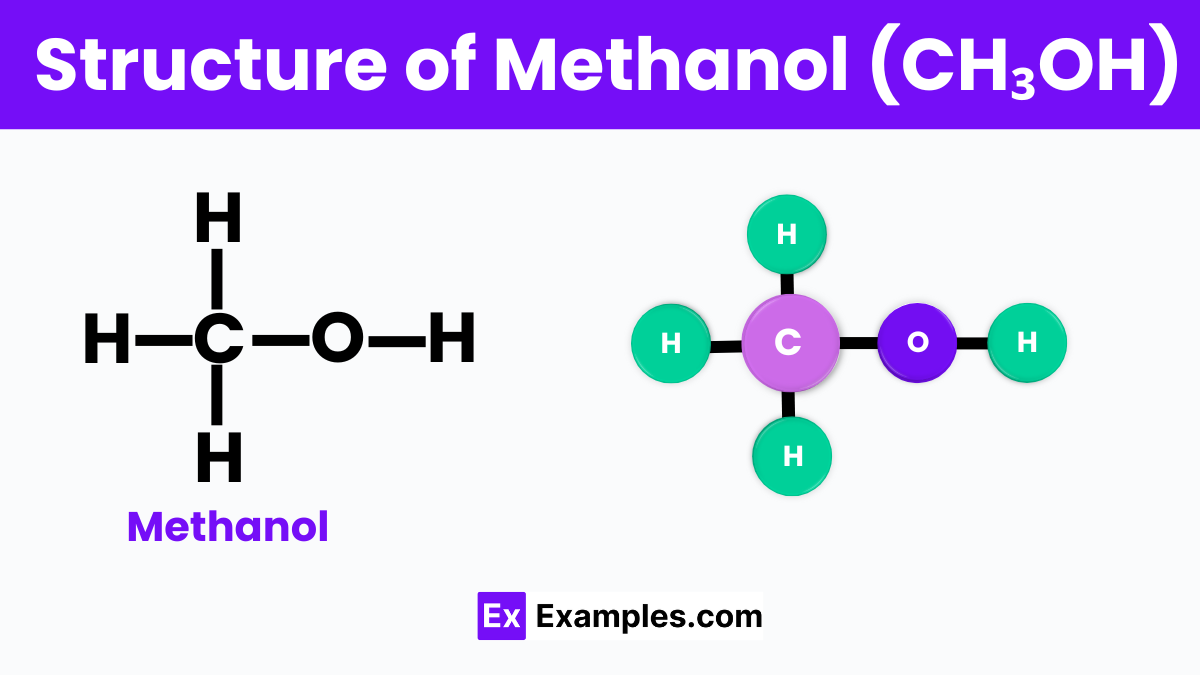
Methanol, also known as wood alcohol, has a simple structure that makes it a key player in the world of chemistry. At its core, methanol is made up of one carbon atom linked to three hydrogen atoms and one oxygen atom that’s also connected to a hydrogen atom. Think of it as a little family, with the carbon atom being the parent and the hydrogen and oxygen atoms as the kids holding hands. This arrangement gives methanol its unique properties, allowing it to mix well with water and be used in a variety of products, from fuel to solvents. Its simple structure is a gateway to understanding more complex chemical compounds, making methanol a fundamental molecule in both science classrooms and industries.
Methanol, a simple but vital chemical, is made through a process that transforms natural gas into something much more versatile. This transformation is like a magic trick; starting with methane, the main component of natural gas, we add a little oxygen to the mix. When methane (CH₄) and oxygen (O₂) are heated up together in the presence of a catalyst—a substance that speeds up the reaction without being consumed—they undergo a chemical change. The end result? Methanol (CH₃OH) and water (H₂O). The chemical equation for this fascinating process looks like this:
In simpler terms, think of it as baking a cake. Just as you mix ingredients like flour, eggs, and sugar to create a delicious treat, chemists mix methane with oxygen under the right conditions to ‘bake’ methanol. This process, known as steam reforming, is widely used in industries around the world to produce methanol on a large scale. Methanol is then used in making various products, from fuels to plastics, showcasing its importance in our daily lives and industries.
| Property | Description |
|---|---|
| Appearance | Methanol is a clear, colorless liquid. It looks a lot like water, so you can’t tell them apart just by looking. |
| Smell | It has a slightly sweet odor, but it’s usually hard to notice unless the concentration is high. |
| Boiling Point | Methanol boils at about 64.7°C (148.5°F). This means it turns into a gas at temperatures lower than boiling water. |
| Freezing Point | It freezes at -97.6°C (-143.7°F), which is way colder than water’s freezing point. So, it stays liquid in extremely cold temperatures. |
| Solubility in Water | Methanol mixes perfectly with water in all proportions. It’s like how sugar dissolves in tea, blending completely. |
| Density | It’s less dense than water, with a density of about 0.791 g/cm³ at 20°C (68°F). This means if you had a bottle of water and a bottle of methanol, the methanol would be lighter. |
Methanol is completely miscible with water, meaning it can be mixed with water in any amount, leading to a clear solution. This property makes methanol an excellent solvent for a wide range of substances, including organic compounds and salts. This solubility plays a critical role in its various applications in industrial and chemical processes.
| Property | Value |
|---|---|
| CAS Registry Number | 67-56-1 |
| Beilstein Number | 1098229 |
| PubChem Compound ID | 887 |
| PubChem Substance ID | 24850836 |
| SMILES Identifier | CO |
| InChI Identifier | InChI=1/CH4O/c1-2/h2H, 1H3 |
| RTECS Number | PC1400000 |
| MDL Number | MFCD00004595 |
| Property | Value |
|---|---|
| NFPA Health Rating | 1 |
| NFPA Fire Rating | 3 |
| NFPA Reactivity Rating | 0 |

Methanol is a clean-burning fuel that can power cars and other vehicles, offering a more environmentally friendly alternative to traditional gasoline. It’s used in special racing cars due to its high octane rating and ability to burn cooler, which improves performance.
In labs around the world, methanol serves as a crucial solvent. Its ability to dissolve a wide range of chemical compounds makes it indispensable for scientists creating medicines, testing samples, or conducting research.
Methanol’s low freezing point makes it an excellent choice for antifreeze products. It helps keep car engines and other machinery running smoothly in cold weather, preventing water-based fluids from freezing.
Thanks to its cleaning power and antifreeze properties, methanol is a key component in windshield washer fluid. It cuts through dirt and grime on car windshields and prevents the fluid from freezing in winter.
Methanol is a primary ingredient in the production of formaldehyde, a chemical used in making building materials, textiles, and many household products. It also serves as a starting point for synthesizing a variety of other important chemicals.
Methanol holds promise as an energy carrier for the future. It can store hydrogen energy, making it easier to transport and use hydrogen fuel, a potential key to unlocking cleaner energy sources.
Yes, methanol is toxic to humans. Ingesting or inhaling it can lead to serious health issues, including blindness, organ failure, and even death.
Methanol is banned in consumer products due to its toxicity. Accidental ingestion or exposure can result in fatal poisoning, prompting strict regulatory measures.
Methanol and ethanol (drinking alcohol) are different. Methanol is toxic and used industrially, while ethanol is safe in moderation and found in beverages.
Beverages like beer and wine have the least methanol, thanks to strict distillation and fermentation processes that minimize methanol content for safe consumption.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary use of phosphoric acid in the methanol industry?
Catalyst
Solvent
Reactant
Stabilizer
Which property makes phosphoric acid suitable as a catalyst in methanol synthesis?
High boiling point
High acidity
Low viscosity
Low reactivity
Phosphoric methanol is primarily used in which type of chemical reactions?
Substitution
Elimination
Esterification
Oxidation
Methanol can be synthesized from which of the following raw materials?
Ethanol
Natural gas
Coal
Both b and c
Which intermediate compound is formed during the synthesis of methanol using phosphoric acid?
Formic acid
Dimethyl ether
Formaldehyde
Ethylene glycol
What is the boiling point of methanol?
56°C
65°C
78°C
100°C
Which safety hazard is associated with methanol?
Corrosive
Flammable
Radioactive
Explosive
Phosphoric acid is often used in methanol synthesis due to its:
Basic nature
Neutral pH
Acidic nature
Amphoteric nature
Which environmental concern is associated with methanol production?
Ozone depletion
Greenhouse gas emission
Water pollution
Acid rain
Methanol is commonly used as a:
Beverage
Fuel
Plasticizer
Lubricant
Before you leave, take our quick quiz to enhance your learning!

