What is the atomic number of Titanium?
20
21
22
23
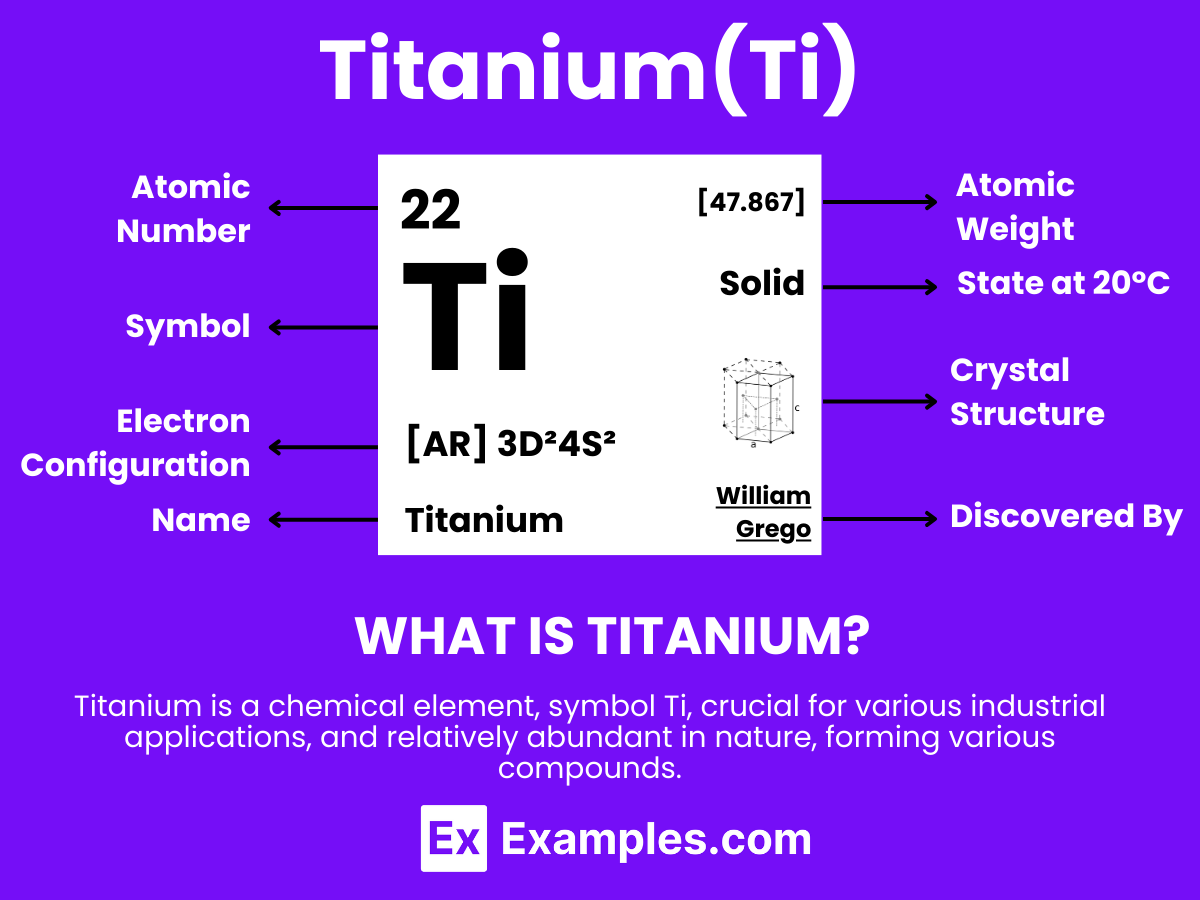
Titanium, a symbol of strength and resilience, stands out in the periodic table for its exceptional properties. This guide embarks on a journey through the world of titanium, showcasing its pivotal role in industries ranging from aerospace to medical implants. With its remarkable strength-to-weight ratio and corrosion resistance, titanium’s applications are as diverse as they are groundbreaking. Through detailed examples, we’ll uncover how this lightweight metal is revolutionizing technology and construction, making the impossible possible. Join us in exploring the multifaceted applications and innovative uses of titanium, a metal that continues to shape the future.
Titanium is a strong, lightweight, silvery-white metallic element known for its outstanding properties and versatility across numerous applications. With the atomic number 22. Titanium is celebrated for its exceptional strength-to-weight ratio and superior resistance to corrosion, including against seawater and chlorine. This element is not found freely in nature but is primarily extracted from rutile and ilmenite ores along with other metals. Titanium’s widespread use spans various fields, notably in the aerospace industry for aircraft, spacecraft, and missiles due to its durability and lightweight properties. Additionally, it’s used in the medical sector for surgical implants and prosthetics, in the sports industry for high-performance equipment, and in architecture for its aesthetic appeal and longevity
The atomic structure of Titanium is a cornerstone in understanding this versatile element’s exceptional characteristics, including its strength, corrosion resistance, and lightweight properties. Titanium, symbolized as Ti and with an atomic number of 22, stands out in the periodic table as a transition metal known for its widespread application across various industries. Here’s a detailed exploration of Titanium’s atomic structure:
| Property | Value |
|---|---|
| Appearance | Silvery-grey, metallic |
| Atomic Number | 22 |
| Atomic Weight | 47.867 |
| Density | 4.506 g/cm³ at 20 °C |
| Melting Point | 1,668 °C (3,034 °F) |
| Boiling Point | 3,287 °C (5,949 °F) |
| State at 20 °C | Solid |
| Electrical Resistivity | 420 nanoohm-meters at 20 °C |
| Thermal Conductivity | 21.9 W/(m·K) at 300 K |
| Heat of Fusion | 14.15 kJ/mol |
| Heat of Vaporization | 425 kJ/mol |
| Specific Heat Capacity | 0.523 J/(g·K) |
| Electronegativity | 1.54 (Pauling scale) |
| Crystal Structure | Hexagonal close-packed (hcp) |
| Magnetic Ordering | Paramagnetic |
Titanium, with its symbol Ti and atomic number 22, is a transition metal known for its strength, low density, and high corrosion resistance. This section delves into the chemical properties of titanium, highlighting its reactivity, common reactions, and significant compounds.
Reactivity: Titanium is less reactive than many metals due to the formation of a passive oxide layer on its surface, which protects it from further corrosion. However, it can react with various elements under certain conditions.
Equation with Oxygen: 2Ti+O₂→2TiO₂
Titanium burns in air to form titanium dioxide (TiO₂), especially at high temperatures, showcasing its ability to bond with oxygen.
Reaction with Acids: Titanium reacts with concentrated acids, illustrating its reactivity with non-oxidizing acids to form titanium(III) chloride (TiCl₃) and hydrogen gas.
Equation with Hydrochloric Acid: Ti+4HCl→TiCl₄ +2H₂
This reaction demonstrates titanium’s ability to dissolve in strong acids, forming tetravalent titanium compounds.
Reaction with Halogens: Titanium reacts vigorously with halogens to form titanium halides, indicating its high reactivity with these elements.
Equation with Chlorine: Ti+2Cl₂→TiCl₄
Titanium tetrachloride (TiCl₄) is a significant compound used in the production of titanium metal and titanium dioxide.
Formation of Alloys: Titanium forms alloys with many other metals, such as aluminum, vanadium, and molybdenum. These alloys are stronger and lighter than pure titanium, making them invaluable in aerospace, military, and sporting goods.
Role as a Catalyst: Titanium compounds, especially titanium dioxide (TiO₂), act as photocatalysts under UV light. This property is exploited in self-cleaning surfaces and in the breakdown of pollutants.
| Property | Value |
|---|---|
| Melting Point | 1668°C (3034°F) |
| Boiling Point | 3287°C (5949°F) |
| Heat of Fusion | 14.15 kJ/mol |
| Heat of Vaporization | 425 kJ/mol |
| Specific Heat Capacity | 25.060 J/(mol·K) |
| Thermal Conductivity | 21.9 W/(m·K) |
| Thermal Expansion | 8.6 µm/(m·K) (at 25°C) |
| Property | Value |
|---|---|
| Atomic Mass | 47.867 u |
| Density | 4.506 g/cm³ (at 20°C) |
| Young’s Modulus | 116 GPa |
| Shear Modulus | 44 GPa |
| Bulk Modulus | 110 GPa |
| Poisson’s Ratio | 0.32 |
| Mohs Hardness | 6 |
| Brinell Hardness | 716 MPa |
| Property | Value |
|---|---|
| Electrical Resistivity | 420 nΩ·m (at 20°C) |
| Magnetic Ordering | Paramagnetic at 300 K |
| Magnetic Susceptibility | +153·10⁻⁶ cm³/mol (at room temperature) |
| Superconducting Point | Below 0.4 K (not naturally occurring) |
| Property | Value |
|---|---|
| Atomic Number | 22 |
| Atomic Weight | 47.867 u |
| Isotopes | Ti-46, Ti-47, Ti-48, Ti-49, Ti-50 |
| Radioactive Isotopes | Ti-44, Ti-45, Ti-51, etc. |
| Half-Lives | Varies from fractions of a second to years for radioactive isotopes |
| Neutron Cross Section | 6.1 barns (for Ti-48) |
| Neutron Mass Absorption | 0.003 (for Ti-48) |
The preparation of titanium is a complex, yet fascinating process that underscores the metal’s value across various high-tech and industrial applications. Titanium is primarily extracted from its most common ores, rutile (TiO₂) and ilmenite (FeTiO₃), through the Kroll process, a method that has stood the test of time for producing high-purity titanium.
Titanium is composed of several isotopes, ranging from those that are stable and naturally occurring to those that are radioactive and synthesized in laboratories. Here is a table that highlights the key isotopes of titanium, including their atomic masses, half-lives, and decay modes when applicable.
| Isotope | Atomic Mass | Half-Life | Mode of Decay |
|---|---|---|---|
| Ti-46 | 46 | Stable | N/A |
| Ti-47 | 47 | Stable | N/A |
| Ti-48 | 48 | Stable | N/A |
| Ti-49 | 49 | Stable | N/A |
| Ti-50 | 50 | Stable | N/A |
| Ti-44 | 44 | 60 years | Electron Capture to Sc-44 |
| Ti-45 | 45 | 184.8 minutes | Beta decay to Sc-45 |
| Ti-51 | 51 | 5.76 minutes | Beta decay to V-51 |
The stable isotopes, particularly Ti-48, make up a significant portion of naturally occurring titanium. The radioactive isotopes, such as Ti-44, have applications in medical imaging and scientific research due to their properties.
Titanium is celebrated for its high strength-to-weight ratio, corrosion resistance, and biocompatibility. These properties make it invaluable across various industries and applications.
The production of Titanium is a sophisticated process that involves several stages, from extraction to refinement, making it a valuable material for various applications. The key steps in the production of Titanium include:
The meticulous production process contributes to the high value and cost of Titanium, reflecting its significance in high-performance and critical applications.
Titanium’s remarkable properties, such as its high strength-to-weight ratio, corrosion resistance, and biocompatibility, have led to its widespread use across numerous industries:
This article has illuminated the exceptional world of titanium, unraveling its vital physical and chemical properties through a concise table. We’ve journeyed from the meticulous preparation processes to its myriad applications, underscoring titanium’s integral role in advancing modern technology and engineering. Titanium’s unparalleled attributes not only define its utility but also forecast a future where its potential is boundlessly explored and utilized.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Titanium?
20
21
22
23
What is the chemical symbol for Titanium?
Ti
Ta
Tm
Te
Titanium is primarily extracted from which mineral?
Hematite
Rutile
Galena
Bauxite
What is the primary use of Titanium in the aerospace industry?
Insulation
Structural components
Paint pigment
Lubricant
What is the molar mass of Titanium?
39.10 g/mol
47.87 g/mol
55.85 g/mol
63.55 g/mol
Which of the following properties makes Titanium ideal for biomedical implants?
High electrical conductivity
Biocompatibility
Low density
Low density
What is the melting point of Titanium?
750°C
1235°C
1668°C
2350°C
Which alloy contains Titanium and is used for its high strength and corrosion resistance?
Brass
Stainless steel
Titanium-aluminum-vanadium alloy (Ti-6Al-4V)
Bronze
Titanium dioxide (TiO$_2$) is commonly used in which product?
Batteries
Sunscreen
Glass
Rubber
Which process is used to extract Titanium from its ores?
Bayer process
Hall-Héroult process
Kroll process
Froth flotation
Before you leave, take our quick quiz to enhance your learning!

