What is the atomic number of Uranium?
90
991
92
93
Uranium, a cornerstone of the nuclear energy sector, embodies a blend of power and complexity. This guide unveils the essence of uranium, exploring its multifaceted roles from fueling atomic reactors to its critical applications in medicine and industry. With a focus on safe extraction, handling, and innovative uses, we dive deep into the science and technology behind uranium’s global influence. Discover the transformative impact of this mighty element, underscoring its significance in advancing sustainable energy solutions and pushing the boundaries of scientific research
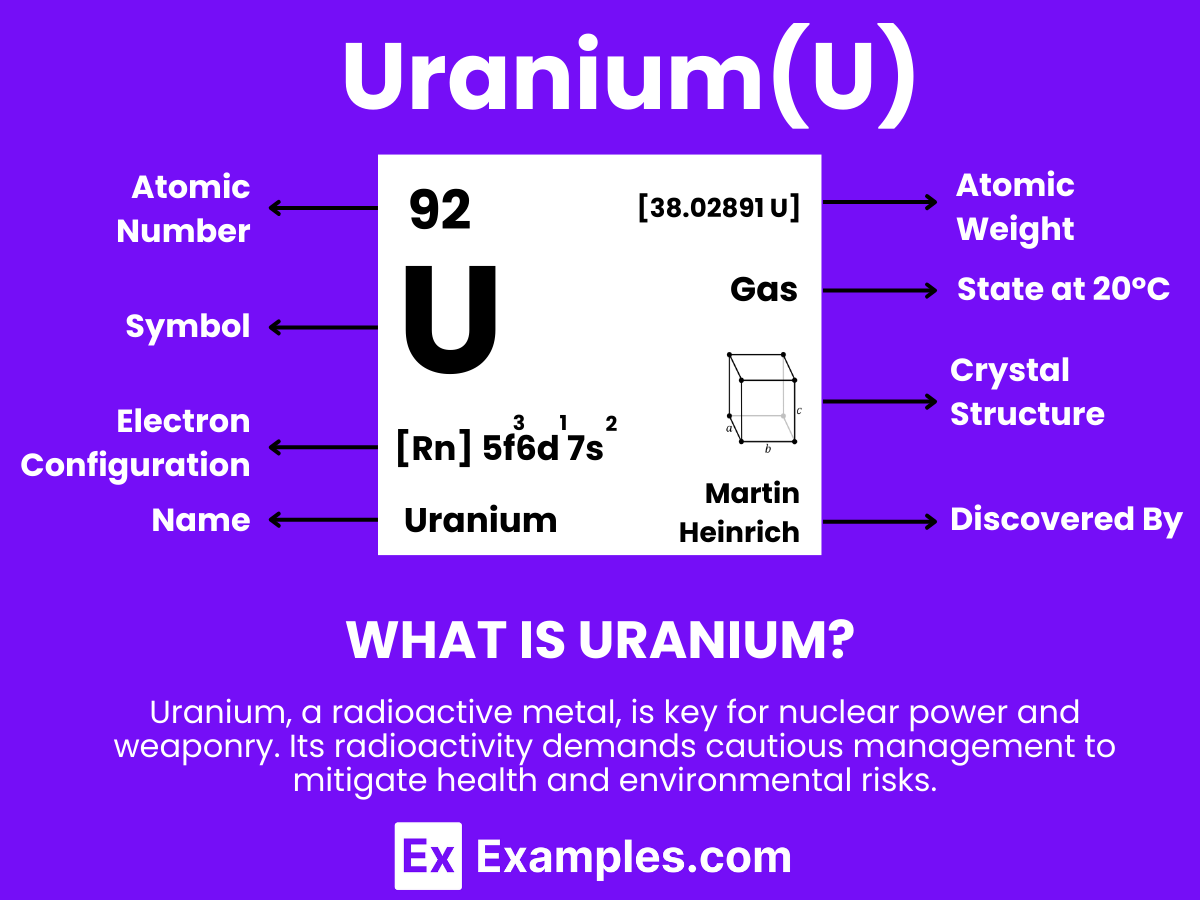
Uranium is a heavy, naturally occurring, radioactive metal with the chemical symbol U and atomic number 92. It stands out for its dense properties and is well-known for its significant role in nuclear energy and weaponry. This element is found in small amounts in rocks, soil, and water and is mined from the Earth’s crust. Uranium is notable for its ability to undergo nuclear fission, a process where its atoms split to release a large amount of energy, which is harnessed in nuclear reactors and atomic bombs. Despite its radioactivity, uranium also has uses in medicine, space exploration, and as a colorant in glass and ceramics.
Uranium, in stark contrast to hydrogen and even gallium, is a heavy metal known for its significant role in nuclear chemistry rather than its occurrence in gaseous form. Its physical and chemical properties are markedly influenced by its position in the periodic table and its actinide nature.
Atomic Level: Each uranium atom (U) contains 92 protons in its nucleus and has 92 electrons orbiting around it. The electron configuration of uranium is [Rn] 5f³ 6d¹ 7s², which highlights it has three electrons in its 5f orbital and one in its 6d orbital available for bonding.
Molecular Formation: Uranium, like gallium, does not form diatomic molecules as hydrogen does (H₂). In its solid state, uranium atoms are arranged in a complex crystalline structure. This arrangement involves metallic bonding, where electrons are shared among a lattice of uranium atoms. Upon melting, uranium transitions to a liquid while maintaining metallic bonding characteristics, which accounts for its high density and surface tension in the liquid state.
Uranium’s lattice bonds are robust, enabling it to preserve its solid state up to its melting point of about 1132.2°C (2070°F). Contrary to hydrogen, which is gaseous at room temperature, uranium remains solid under standard conditions and melts at temperatures significantly higher than room temperature. Given its very high boiling point of approximately 4131°C (7468°F), uranium does not naturally exist as a gas or in a diatomic gaseous state under normal conditions. The concept of “Uranium Gas” primarily relates to uranium hexafluoride (UF6), a compound used in the gas phase for uranium enrichment processes, rather than elemental uranium in a gaseous state.
| Property | Description |
|---|---|
| Appearance | Silvery-gray metallic |
| Atomic Number | 92 |
| Density (at 20°C) | 19.1 g/cm³ |
| Melting Point | 1132.2°C (2070°F) |
| Boiling Point | 4131°C (7468°F) |
| State at Room Temperature | Solid |
| Electron Configuration | [Rn]5f36d17s2 |
| Common Oxidation States | +3, +4, +6 |
Uranium exhibits several chemical properties that are significant both in nature and in various applications, particularly in nuclear science.
| Property | Value with Unit |
|---|---|
| Boiling Point | 4131 °C |
| Melting Point | 1132.2 °C |
| Critical Temperature | Not Available |
| Critical Pressure | Not Available |
| Heat of Vaporization | 417.1 kJ/mol |
| Heat of Fusion | 9.14 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.116 J/g·K |
| Thermal Conductivity | 27.5 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 20°C) | 19.1 g/cm³ |
| Viscosity | Not Applicable (Solid) |
| Solubility in Water | Insoluble |
| Color | Metallic silver to dull gray |
| Phase at Room Temperature | Solid |
| Property | Value with Unit |
|---|---|
| Electrical Resistivity (at 0°C) | 0.28 µΩ·m |
| Thermal Conductivity | 27.5 W/m·K |
| Magnetic Susceptibility | +40.9 × 10^-6 cm^3/mol |
| Electronegativity (Pauling scale) | 1.38 |
| Property | Value with Unit |
|---|---|
| Atomic Number | 92 |
| Atomic Mass | 238.02891 u |
| Isotopes | ^238U (99.2745%), ^235U (0.720%), ^234U (0.0055%) |
| Half-Life (for ^238U) | 4.468 × 10^9 years |
| Half-Life (for ^235U) | 7.04 × 10^8 years |
| Half-Life (for ^234U) | 245,500 years |
| Nuclear Spin (for ^235U) | 7/2 ℏ |
| Neutron Cross Section (for ^238U) | 2.68 barns |
| Neutron Cross Section (for ^235U) | 681 barns (thermal neutron) |
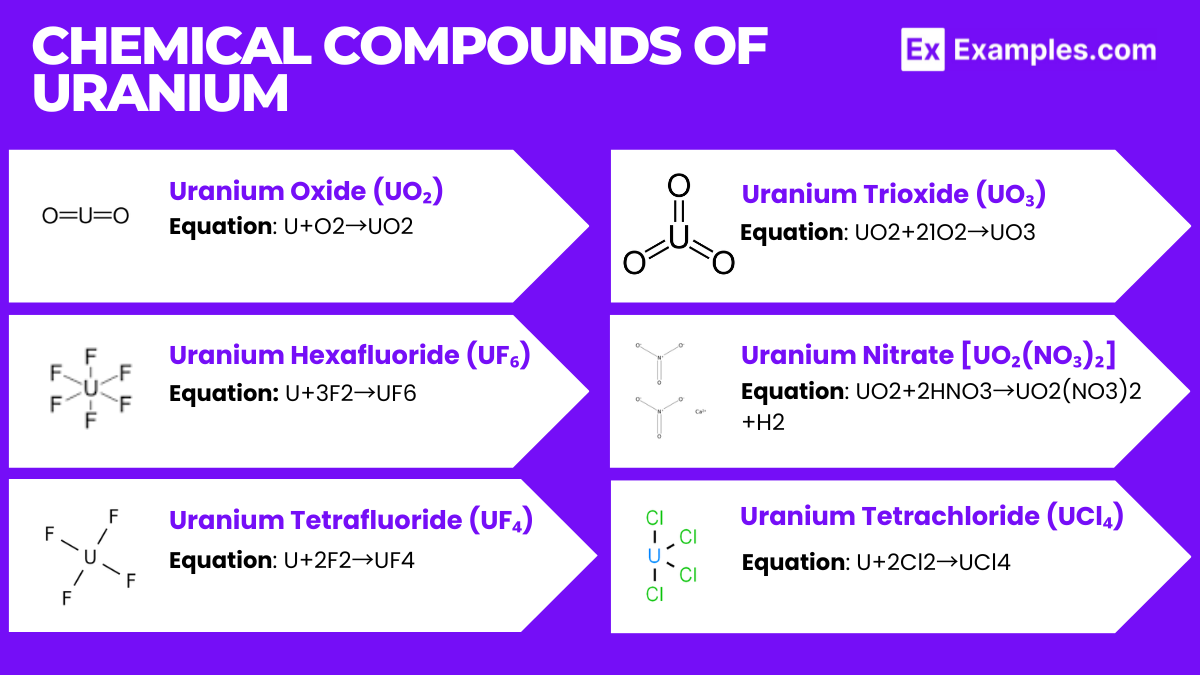
The preparation of uranium from its ores involves several complex processes that extract and purify uranium. This multi-step process is essential for producing uranium suitable for use in nuclear reactors and weapons.
Spent nuclear fuel and other uranium-containing wastes are handled with care due to their radioactivity and potential environmental impact. Reprocessing spent fuel can recover unused uranium and plutonium, which can be reused as fuel, although this process is complex and involves significant safety and non-proliferation considerations.
The preparation of uranium is a highly technical and regulated process, reflecting its importance in energy production and national security, as well as the need for careful management of radioactive materials.
Uranium has several isotopes, each with unique properties and applications. The most significant isotopes of uranium include:
Uranium’s uses are diverse, ranging from energy production to military applications and scientific research:
The production of uranium involves several key steps to extract, process, and refine the raw uranium ore into a usable form for energy production, military applications, and other uses. Here’s an overview of the uranium production process:
Uranium’s primary applications are centered around its energy-producing capabilities and its density. Here are some of the key uses:
In summary, uranium plays a pivotal role in modern society, primarily fueling nuclear energy production and national defense through its use in nuclear weapons. Its unique properties, from high density to radioactive nature, also find applications in medicine, industry, and science. The careful extraction, processing, and management of uranium underscore its significance and the need for responsible stewardship.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Uranium?
90
991
92
93
What is the chemical symbol for Uranium?
Ur
U
Un
Urn
Which isotope of Uranium is commonly used as fuel in nuclear reactors?
U-234
U-235
U-236
U-238
What is the most common oxidation state of Uranium in its compounds?
+2
+3
+4
+6
Uranium is primarily obtained from which mineral?
Hematite
Bauxite
Galena
Uraninite
What is the primary use of Uranium in the modern world?
Weaponry
Construction
Nuclear fuel
Electronics
What is the half-life of Uranium-238?
4.5 billion years
1 million years
100,000 years
700 million years
Which process is used to enrich Uranium for use in nuclear reactors?
Electrolysis
Fractional distillation
Gas centrifugation
Froth flotation
Which of the following is a characteristic of Uranium metal?
Brittle
Dense and heavy
Highly reactive with water
Transparent
What color does Uranium glass typically exhibit under UV light?
Blue
Red
Green
Yellow
Before you leave, take our quick quiz to enhance your learning!

