Vanadium is primarily used in the production of:
Aluminum
Stainless steel
Titanium
Brass
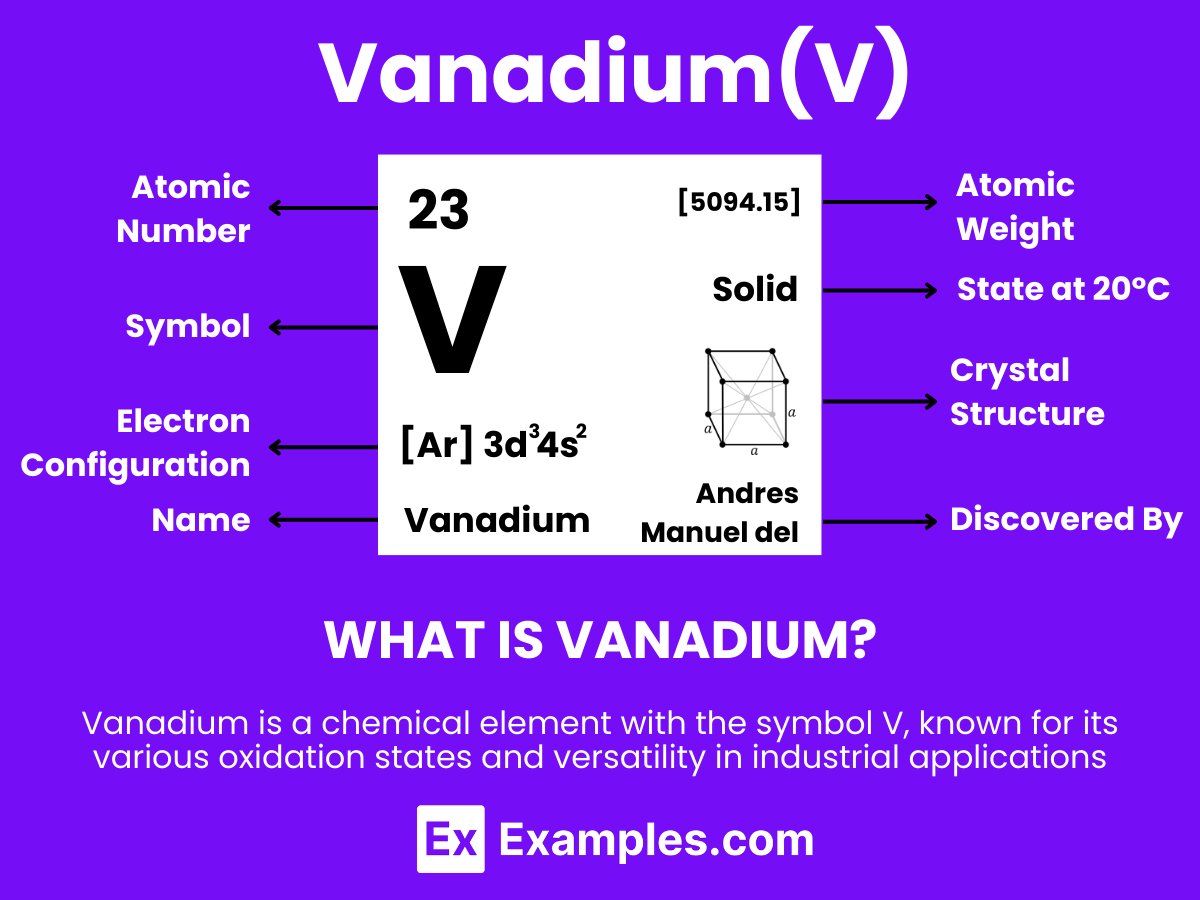
Discover the versatile world of Vanadium, a remarkable element that bridges the gap between technology and innovation. This complete guide delves into the essence of Vanadium, highlighting its unique properties, applications, and the pivotal role it plays in modern advancements. From strengthening steel alloys to revolutionizing energy storage in batteries, Vanadium’s impact is profound. Uncover the myriad ways this dynamic element enhances our lives through practical examples, showcasing its significance in today’s fast-paced world.
Vanadium is a tough, silvery-grey metallic element that stands out due to its exceptional characteristics and broad application spectrum. With the atomic number 23. Vanadium is renowned for its impressive strength, ability to resist corrosion, and its role as a hardening agent in steel alloys. Unlike ruthenium, vanadium is more abundantly found in nature and is primarily extracted from magnetite, vanadinite, and other minerals. Additionally, vanadium is pivotal in the production of certain alloys and in the chemical industry as a catalyst for sulfuric acid production. In energy storage, vanadium redox flow batteries are gaining attention for their potential in renewable energy storage solutions, highlighting the element’s versatility and importance in modern technological advancements.
Vanadium, with the chemical symbol V and atomic number 23, is a transition metal known for its unique properties and applications. Understanding its atomic structure provides insight into its behavior and uses in various industries.
Isotopes: Vanadium primarily exists in nature as Vanadium-51 (stable isotope). There are also several other isotopes with varying numbers of neutrons, exhibiting different stability levels.
Valence Electrons: The valence electrons for vanadium are the five electrons in the 3d and 4s orbitals. These electrons play a pivotal role in chemical reactions and bonding, as they can be lost, gained, or shared with other atoms to form chemical compounds.
Shells and Orbitals: The electron shells of vanadium are filled in the order of increasing energy levels, starting from the closest to the nucleus. Vanadium’s electrons fill the 1s, 2s, 2p, 3s, 3p, 4s, and 3d orbitals, in that sequence, following the principles of electron configuration.
Chemical Bonding: Vanadium can exhibit multiple oxidation states, most commonly +2, +3, +4, and +5. These oxidation states reflect the number of electrons vanadium can donate or share during chemical reactions, leading to a variety of compounds with different properties.
Role in Compounds: Vanadium forms a range of compounds, including oxides, sulfides, and various organometallic compounds. Its ability to exist in multiple oxidation states makes it versatile in catalysis, redox reactions, and as an alloying element to enhance the strength and durability of metals.
Below is a table detailing the key physical properties of vanadium, a versatile and valuable metallic element known for its unique combination of durability, malleability, and resistance to corrosion.
| Property | Description |
|---|---|
| Atomic Number | 23 |
| Atomic Mass | 50.9415 u |
| Density | 6.0 g/cm³ at 20°C |
| Melting Point | 1910°C |
| Boiling Point | 3407°C |
| State at Room Temperature | Solid |
| Color | Silvery-grey metallic |
| Crystal Structure | Body-centered cubic (BCC) |
| Electrical Conductivity | Moderate, metallic conductor |
| Thermal Conductivity | Good, facilitating its use in high-temperature applications |
| Property | Value | Unit |
|---|---|---|
| Melting Point | 2183 | K (1910 °C) |
| Boiling Point | 3680 | K (3407 °C) |
| Heat of Fusion | 21.5 | kJ/mol |
| Heat of Vaporization | 459 | kJ/mol |
| Specific Heat Capacity | 24.89 | J/(mol·K) |
| Thermal Conductivity | 30.7 | W/(m·K) |
| Thermal Expansion | 8.4 | µm/(m·K) |
| Property | Value | Unit |
|---|---|---|
| Density | 6.0 | g/cm³ |
| Young’s Modulus | 128 | GPa |
| Tensile Strength | 690 | MPa |
| Hardness (Vickers) | 628 | HV |
| Poisson’s Ratio | 0.37 | – |
| Elastic Modulus | 128 | GPa |
| Property | Value | Unit |
|---|---|---|
| Electrical Resistivity | 197 | nΩ·m |
| Magnetic Ordering | Paramagnetic | – |
| Superconducting Point | Below 5.4 | K |
| Thermal Conductivity | 30.7 | W/(m·K) |
| Property | Value |
|---|---|
| Natural Isotopes | V-50, V-51 |
| Stable Isotopes | V-51 |
| Unstable Isotopes | V-50 and others |
| Abundance in Nature | V-50: 0.25%, V-51: 99.75% |
| Atomic Number | 23 |
| Neutron Cross Section | 5.08 barns (for V-51) |
| Neutron Mass Absorption | 0.017 (for V-51) |
| Isotopic Mass | V-50: 49.94716 u, V-51: 50.94396 u |
Vanadium ores are first crushed and ground to liberate the vanadium-bearing minerals from the host rock. The ground ore is then subjected to various processes to increase the concentration of vanadium. This can involve physical methods such as flotation and magnetic separation.
The concentrated vanadium ore undergoes roasting with sodium carbonate or sodium chloride, which transforms the vanadium into soluble forms. Following roasting, the ore is leached with water or acid, which results in the formation of a vanadium-bearing solution. This solution contains vanadium in the form of vanadates or vanadium oxides.
The vanadium is then precipitated from the solution. This can be achieved through various methods, such as adding ammonium sulfate to convert vanadium into ammonium metavanadate (NH_4VO_3), which precipitates out of the solution.
The precipitated ammonium metavanadate is then calcined (heated) in the presence of air. This process converts the ammonium metavanadate into vanadium pentoxide (V_2O_5), a powdery yellow compound, which is the most commercially significant form of vanadium.
For applications that require pure vanadium, the vanadium pentoxide is further reduced. This is usually achieved through a process involving aluminum (Al) or silicon (Si) at high temperatures. The reaction reduces the vanadium pentoxide to metallic vanadium.
In some cases, further purification is required, which can be achieved through electrolytic refining. This process involves dissolving vanadium pentoxide in an acid and then applying an electric current to deposit pure vanadium at the cathode.
The final product is a pure vanadium metal or vanadium compounds, depending on the intended application. Vanadium is used in various applications, including as an additive in steel to increase its strength and resistance to corrosion and wear, in chemical catalysts, and in batteries.
Vanadium, a transition metal, forms various compounds with distinct properties and applications. Here are six chemical compounds of vanadium, described in brief with their relevant equations:
| Isotope | Symbol | Atomic Mass | Half-Life | Stability | Natural Occurrence |
|---|---|---|---|---|---|
| 48 | V-48 | 47.9522537 | Stability: Stable | Stable | Trace |
| 49 | V-49 | 48.9485161 | Stability: Stable | Stable | Trace |
| 50 | V-50 | 49.9471585 | Stability: Stable | Stable | 0.25% |
| 51 | V-51 | 50.9439595 | Stability: Stable | Stable | 99.75% |
Note: This table includes the most common isotopes of vanadium, focusing on those that are stable or occur naturally. Vanadium has one stable isotope (V-51) that makes up the majority of natural vanadium. Other isotopes exist but are either synthetic or found in trace amounts.
Vanadium is a versatile element with a variety of applications due to its unique properties, including strength, resistance to corrosion, and stability at high temperatures. Here are some of the primary uses of vanadium:
Vanadium production involves multiple steps from extraction to purification:
Vanadium is utilized in various industries due to its unique properties:
vanadium plays a critical role across various industries, notably in strengthening steel and in energy storage solutions. Its intricate extraction and refinement process underscores the metal’s value and versatility. As we advance, the efficient production and innovative applications of vanadium will continue to be pivotal in meeting global technological and material science challenges
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Vanadium is primarily used in the production of:
Aluminum
Stainless steel
Titanium
Brass
Vanadium naturally occurs in which type of minerals?
Oxides
Sulfides
Sulfides
Carbonates
What is the melting point of vanadium?
1670°C
1910°C
2183°C
2500°C
Which vanadium compound is used as a catalyst in the production of sulfuric acid?
Vanadium(II) oxide
Vanadium(III) oxide
Vanadium(IV) oxide
Vanadium(V) oxide
Vanadium alloys are known for their:
High density
Low melting point
High tensile strength
Poor conductivity
Vanadium is classified as a:
Alkali metal
Alkaline earth metal
Transition metal
Lanthanide
Vanadium is important in biological systems because it is:
A cofactor for enzymes
A component of DNA
Used in muscle contraction
Involved in blood clotting
What is the density of vanadium?
5.96 g/cm³
6.11 g/cm³
6.95 g/cm³
7.24 g/cm³
Vanadium is resistant to:
Acid corrosion
Base corrosion
Oxidation
Thermal expansion
Which of the following processes is used to extract vanadium from its ores?
Electrolysis
Carbon reduction
Hydrometallurgy
Froth flotation
Before you leave, take our quick quiz to enhance your learning!

