What does the Boltzmann distribution describe?
Distribution of particle velocities in a gas
Distribution of charges in a conductor
Distribution of masses in a galaxy
Distribution of wavelengths in a blackbody spectrum


The Boltzmann Distribution is a fundamental concept in statistical mechanics, providing a powerful means to describe how particles, such as atoms or molecules, are distributed among various energy states in thermal equilibrium. This distribution is crucial for understanding a wide range of physical phenomena, including the behavior of gases and the principles underlying the Laws of Fluid Dynamics.
The Boltzmann Distribution Formula is a simple yet powerful expression. It quantifies the probability of particles occupying different energy states in thermal equilibrium. It is represented as:
Here, P(E) denotes the probability of finding a particle in an energy state E, k is the Boltzmann constant, and T represents the absolute temperature. This formula shows that the likelihood of a particle being in a certain energy state. It decreases exponentially with increasing energy and is more favorable at higher temperatures.
The Boltzmann Distribution can be understood and derived through a straightforward conceptual approach, focusing on the behavior of particles in a system at thermal equilibrium without delving into complex equations.
Consider a system where particles, such as atoms or molecules, can exist in various energy states. These energy states could range from low to high energy levels.
When the system reaches thermal equilibrium, it means the temperature is uniform throughout, and the energy distribution among the particles is stable over time. At this point, no net energy transfer occurs between different parts of the system.
In a naturally occurring scenario, particles tend to favor lower energy states because these are more stable and less energy-intensive to maintain. However, with an increase in temperature, particles gain more thermal energy, which allows them to reach and maintain higher energy states.
The probability of finding a particle in a higher energy state decreases exponentially as the energy increases. This is because it’s statistically less likely for particles to achieve and sustain higher energy levels without additional energy input (like an increase in temperature).
At equilibrium, the distribution of particles among the energy states follows a pattern where most particles will be found in the lower energy states, and fewer will populate the higher states, creating a balance dictated by the temperature of the system.
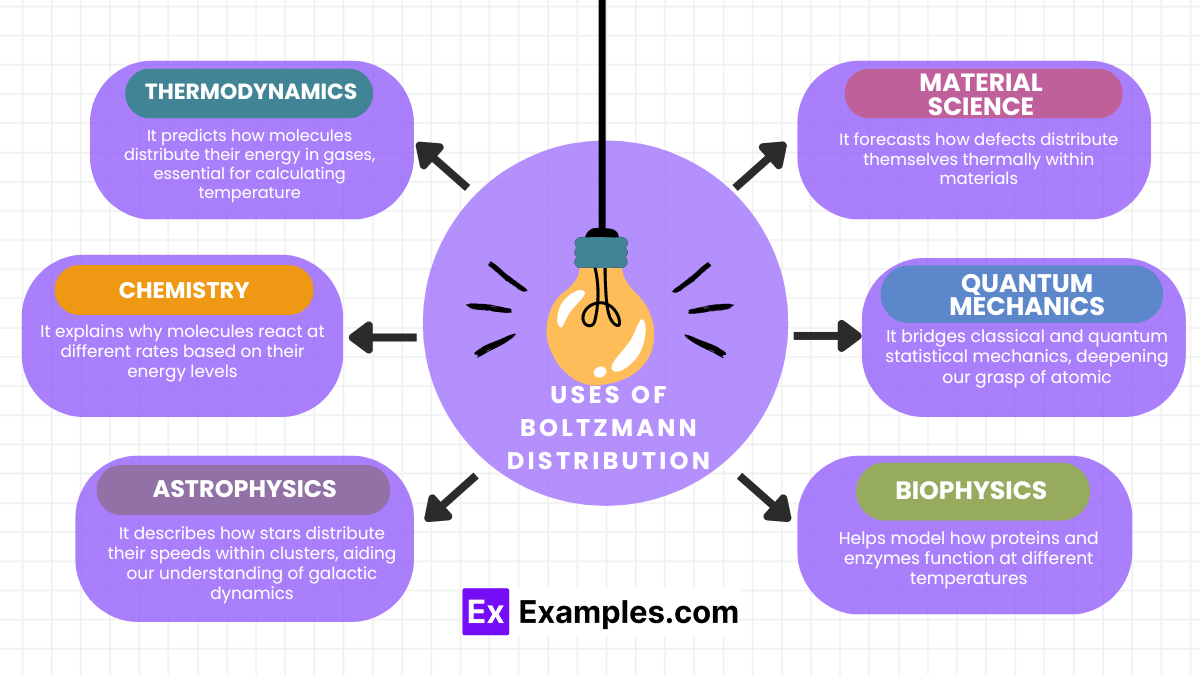
The Boltzmann Distribution plays a pivotal role across various scientific disciplines. Here’s how it actively contributes:
The Boltzmann Distribution provides critical insights across a spectrum of scientific fields. Here are some illustrative examples, enhanced with transition words for clearer understanding:
Named after Ludwig Boltzmann, it quantifies the relationship between kinetic energy and temperature in gases, reflecting his contributions to statistical mechanics.
Gibbs distribution applies to systems with varying particle numbers and energy. Whereas Boltzmann distribution specifically addresses systems at constant temperature and particle count.
Temperature significantly influences the Maxwell-Boltzmann distribution, as higher temperatures increase the kinetic energy, thereby broadening the distribution of particle speeds.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does the Boltzmann distribution describe?
Distribution of particle velocities in a gas
Distribution of charges in a conductor
Distribution of masses in a galaxy
Distribution of wavelengths in a blackbody spectrum
In the context of the Boltzmann distribution, what does 'T' represent?
Time
Temperature
Tension
Torque
What happens to the Boltzmann distribution as the temperature increases?
It becomes narrower
It shifts to higher energy states
It shifts to lower energy states
It remains the same
What is the main principle behind the Boltzmann distribution?
Conservation of energy
Equipartition of energy
Distribution of particles among energy states
Conservation of mass
How is the Boltzmann distribution related to entropy?
Higher entropy corresponds to a narrower distribution
Higher entropy corresponds to a wider distribution
Entropy is independent of the distribution
Lower entropy corresponds to a wider distribution
Which of the following best describes the shape of the Boltzmann distribution curve at high temperatures?
Symmetrical
Narrow and peaked
Broad and flattened
Inverted
What does the factor 'kT' represent in the Boltzmann distribution equation?
Kinetic energy
Potential energy
Thermal energy
Binding energy
How does the Boltzmann distribution explain the behavior of gases?
It explains the distribution of molecular speeds in a gas
It describes the pressure exerted by gases
It explains why gases expand to fill their container
It describes the volume of gases
In the Boltzmann distribution, what does 'E' represent?
Energy of a particle
Electric field strength
Elastic modulus
Efficiency
What is the effect of decreasing temperature on the Boltzmann distribution?
It shifts to higher energy states
It shifts to lower energy states
It becomes wider
It becomes more uniform
Before you leave, take our quick quiz to enhance your learning!

