What does Fick's first law of diffusion describe?
The rate of diffusion
The concentration gradient
The diffusion coefficient
The molar flux

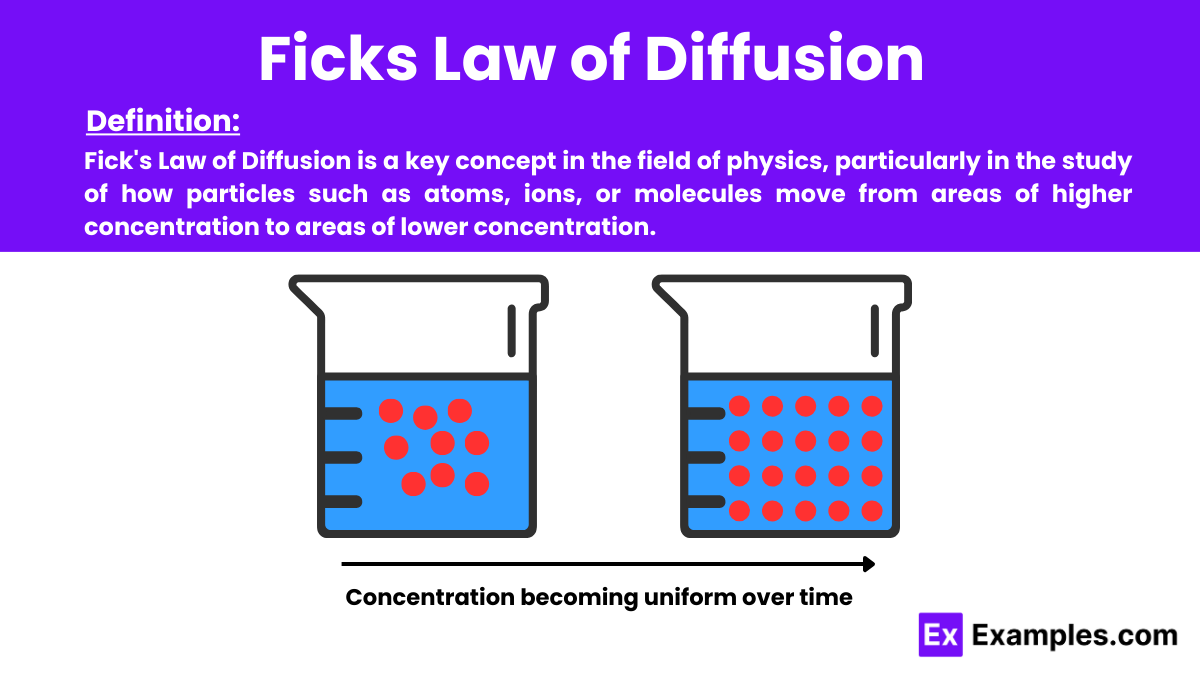
Fick’s Law of Diffusion is a key concept in the field of physics, particularly in the study of how particles such as atoms, ions, or molecules move from areas of higher concentration to areas of lower concentration. This movement is driven by a gradient in concentration, and the law quantifies this diffusion process, which is essential in understanding various natural and engineered systems.
Fick’s First Law quantifies the rate at which particles diffuse across a medium, providing a foundational concept in the study of diffusion processes. It essentially states that the diffusion flux is directly proportional to, and flows in the direction opposite to, the concentration gradient.
The law defines diffusion flux, 𝐽, as the amount of substance that passes through a unit area per unit time. It indicates that this flux occurs from regions of higher concentration to regions of lower concentration.
Mathematically, Fick’s First Law is:
Fick’s Second Law states that the rate of change of concentration with respect to time is proportional to the spatial derivative of the concentration gradient. This indicates that diffusion causes the concentration distribution to evolve, seeking equilibrium across the medium.
The mathematical expression of Fick’s Second Law is:
Fick’s Law of Diffusion is crucial for understanding how particles move from high to low concentration areas. This simple yet powerful law is key in many fields:
Overall, Fick’s Law is essential for any application where materials mix or react, providing a foundation for innovation and efficient design across various industries.
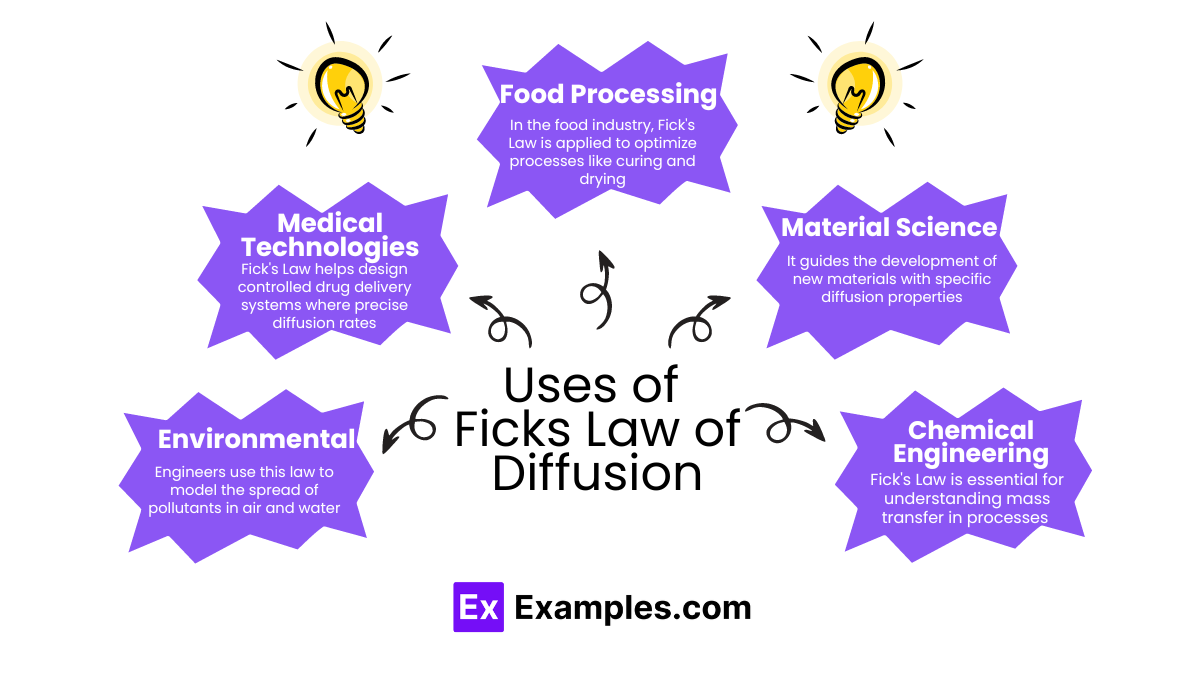
Fick’s Law of Diffusion is instrumental in several areas, providing insights into how substances move through various mediums. Here are some key applications:
No, Fick’s law is not a gas law; it specifically addresses diffusion processes in liquids, gases, and solids, focusing on concentration gradients.
Yes, Fick’s law can apply to osmosis to some extent, as it helps describe how solvent molecules move through a semipermeable membrane from lower to higher solute concentrations.
Several factors impact Fick’s law: the diffusion coefficient, the concentration gradient, and the temperature. Each altering how quickly and efficiently diffusion occurs.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does Fick's first law of diffusion describe?
The rate of diffusion
The concentration gradient
The diffusion coefficient
The molar flux
In Fick's first law, what does the diffusion flux (J) represent?
Amount of substance per unit area per unit time
Change in concentration
Total amount of substance diffused
Time taken for diffusion
According to Fick's first law, how is the diffusion flux (J) related to the concentration gradient (dC/dx)?
J = D dC/dx
J = D dC*dx
J = dC/D dx
J = dC dx/D
What is the unit of the diffusion coefficient (D) in Fick's law?
m²/s
m/s²
m³/s
s/m²
How does temperature affect the diffusion coefficient (D) in Fick's law?
D increases with temperature
D decreases with temperature
D remains constant
D becomes zero
What does Fick's second law of diffusion describe?
Steady-state diffusion
Time-dependent diffusion
Concentration gradient
Diffusion flux
In Fick's second law, which variable represents time?
t
x
C
J
How is Fick's second law derived from Fick's first law?
By differentiating the first law with respect to time
By integrating the first law with respect to distance
By multiplying the first law by the diffusion coefficient
By dividing the first law by the concentration gradient
In the context of Fick's laws, what is the significance of a negative diffusion flux?
Substance is moving from high to low concentration
Substance is moving from low to high concentration
Diffusion has stopped
Diffusion coefficient is negative
Which of the following factors does NOT affect the diffusion coefficient (D)?
Temperature
Medium through which diffusion occurs
Concentration gradient
Molecular size
Before you leave, take our quick quiz to enhance your learning!

