What does the Law of Equipartition of Energy state?
Energy is conserved in an isolated system
Energy is equally distributed among all degrees of freedom
Energy is lost as heat in any process
Energy is transferred as work in any process


The Law of Equipartition of Energy is a fundamental principle in statistical mechanics. A branch of physics that deals with the behavior of systems with a large number of particles. This law provides crucial insights into how energy is distributed among the degrees of freedom of a system at thermal equilibrium.
The Law of Equipartition of Energy primarily describes how energy distributes equally among the degrees of freedom in a system at thermal equilibrium. This principle manifests in different contexts, explaining various physical phenomena.
The classical version of the equipartition theorem states that each degree of freedom that appears quadratically in the energy expression contributes 1/2𝑘𝑇 to the average energy of the system. Where 𝑘 is Boltzmann’s constant, and 𝑇 is the temperature in Kelvin.
In quantum mechanics, quantization of energy levels causes the equipartition theorem to apply differently. The energy distribution among the degrees of freedom depends on the temperature. Relative to the energy quantum of the mode. Thus, the classical equipartition theorem only applies at high temperatures. Where the thermal energy greatly exceeds the quantum of energy for the mode.
The law states that each degree of freedom that contributes quadratically to the system’s energy receives an average energy of 1/2𝑘𝑇, where:
In general terms, for a system at thermal equilibrium, the total energy 𝐸E is distributed equally across all its degrees of freedom, and the expression becomes:
In classical statistical mechanics, we often describe the total energy of a system using the Hamiltonian, which may include various types of energy such as kinetic and potential. We can express the Hamiltonian as a function of the particles’ positions and momenta in the system.
Consider a system described by the Hamiltonian 𝐻(𝑝,𝑞), where p represents the generalized momenta and q represents the generalized coordinates of the particles in the system. The system is assumed to be at thermal equilibrium, and the particles follow the Maxwell-Boltzmann distribution.
The probability 𝑃P that the system is in a particular state with energy E is given by the Boltzmann factor:
where 𝑘 is the Boltzmann constant and 𝑇 is the temperature of the system.
To find the average energy associated with a specific degree of freedom, integrate over all phase space while keeping one variable, say a component of the momentum 𝑝𝑖, fixed:
This integral focuses on the kinetic part of the energy for one degree of freedom associated with the momentum 𝑝𝑖.
The integral simplifies using Gaussian integration techniques, where the average kinetic energy associated with one component of the momentum is:
This expression states that the average kinetic energy per degree of freedom is 1/2𝑘𝑇.
Since the derivation can be applied to any quadratic degree of freedom (either in kinetic or potential form), each degree of freedom contributes 1/2𝑘𝑇 to the total energy. Therefore, if a system has 𝑓 degrees of freedom, the total energy at equilibrium is:
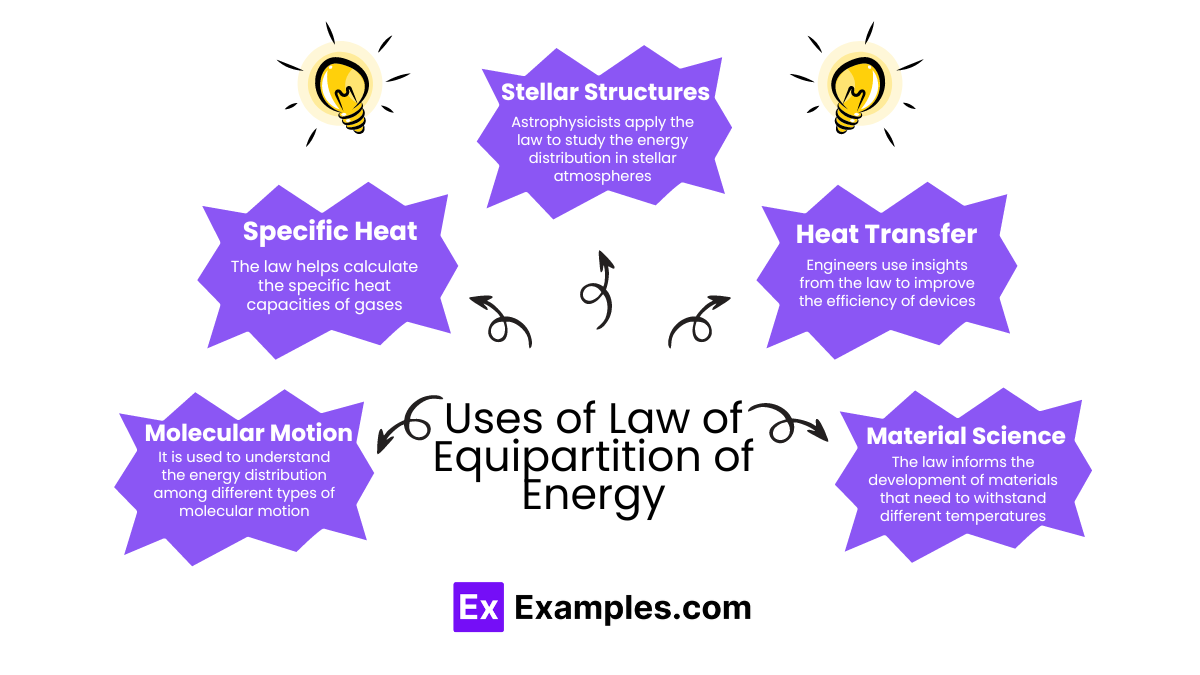
The Law of Equipartition of Energy is fundamental in physics and has broad applications across various scientific fields. Here are some key uses:
The Law of Equipartition of Energy provides valuable insights across various applications. Here are some specific examples illustrating its utility:
The principle of equipartition states that energy distributes equally among all available degrees of freedom at thermal equilibrium in a system.
In kinetic theory, the equipartition of energy principle explains that each degree of freedom. In a gas molecule contributes equally to its kinetic energy.
James Clerk Maxwell and Ludwig Boltzmann developed the law of equipartition of energy. Elaborating on how energy equilibrates in thermodynamic systems.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does the Law of Equipartition of Energy state?
Energy is conserved in an isolated system
Energy is equally distributed among all degrees of freedom
Energy is lost as heat in any process
Energy is transferred as work in any process
According to the Law of Equipartition of Energy, how much energy is associated with each degree of freedom at temperature T?
kT/2
kT
2kT
kT/4
How many degrees of freedom does a monatomic gas molecule have?
3
5
6
7
What is the total energy of a monatomic gas molecule at temperature T according to the Law of Equipartition of Energy?
3kT
3kT/2
2kT
kT
How many degrees of freedom does a diatomic gas molecule have at room temperature?
1
2
3
5
According to the Law of Equipartition of Energy, what is the average energy of a diatomic gas molecule at temperature T?
5kT/2
3kT/2
kT
7kT/2
What additional degrees of freedom do polyatomic gases have compared to monatomic gases?
Translational
Rotational
Vibrational
Both rotational and vibrational
For a system in thermal equilibrium, what determines the distribution of energy among its degrees of freedom?
Temperature
Pressure
Volume
Mass
What is Boltzmann's constant (k) used for in the Law of Equipartition of Energy?
It relates pressure to volume
It relates temperature to energy
It relates energy to mass
It relates volume to mass
In the context of the Law of Equipartition of Energy, what is a degree of freedom?
A specific direction of motion
A specific type of potential energy
A specific mode in which a system can possess energy
A specific type of kinetic energy
Before you leave, take our quick quiz to enhance your learning!

