What is the molecular formula of ammonia?
NH₂
NH₃
N2H4
NH₄

Ammonia is a fascinating player in the world of chemistry, known for its sharp smell and clear, colorless gas form. As a covalent compound, it’s formed when nitrogen and hydrogen atoms share electrons tightly, bonding them together in a unique and stable way. This simple yet intriguing substance plays a crucial role in both nature and industry, showcasing the amazing versatility of chemical compounds.
| Property | Value |
|---|---|
| Formula | NH₃ |
| Hill formula | H₃N |
| Name | Ammonia |
| Alternate names | Ammonia anhydrous, Ammonia gas, Azane, Nitro-sil, Spirit of Hartshorn |
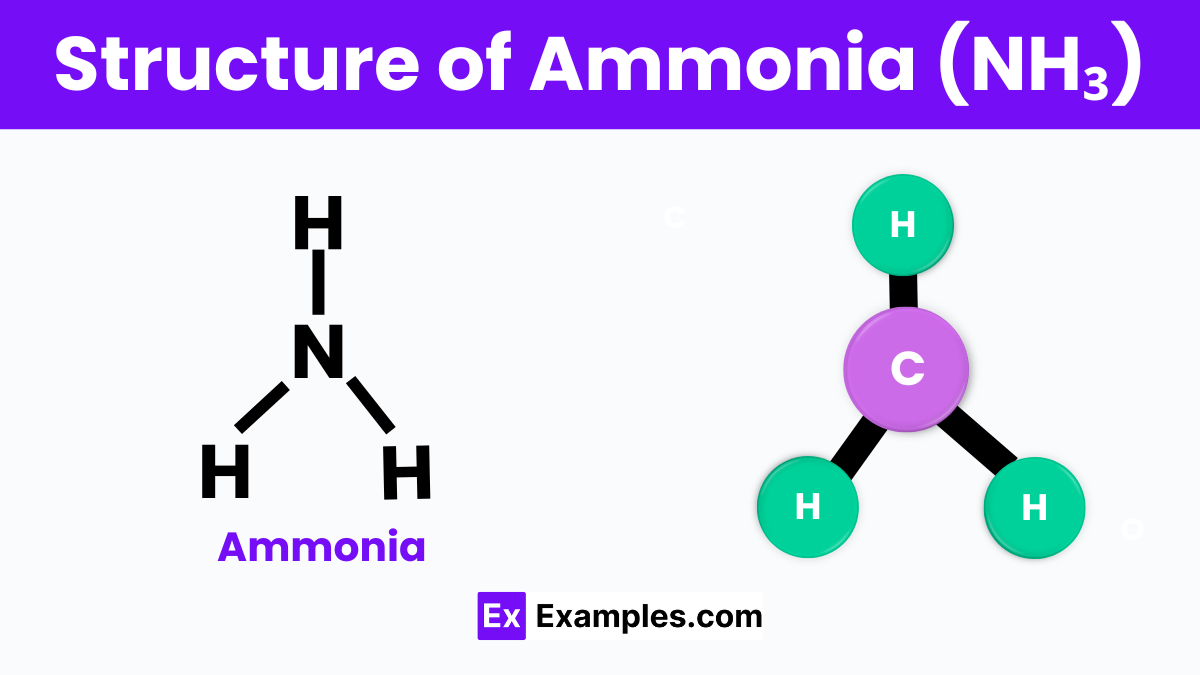
Ammonia is a compound made up of nitrogen and hydrogen with the formula NH3. This means that one molecule of ammonia contains one nitrogen atom and three hydrogen atoms. The structure of ammonia is shaped like a pyramid. At the top of this pyramid is the nitrogen atom, and the three hydrogen atoms form the base. This shape is often referred to as a trigonal pyramidal structure. The nitrogen atom has five electrons in its outer shell, but it shares three of these electrons with three hydrogen atoms to form three covalent bonds. This sharing of electrons allows the ammonia molecule to have a stable structure. Additionally, the nitrogen atom has a lone pair of electrons that are not shared with the hydrogen atoms, which contributes to the molecule’s shape and properties.
Ammonia is commonly prepared in
the industry through a process called the Haber-Bosch process. This process involves combining nitrogen gas (N2) from the air with hydrogen gas (H2) under high pressure and temperature in the presence of a catalyst. A catalyst is a substance that helps speed up a chemical reaction without being used up in the process. The high pressure and temperature conditions, along with the catalyst, help break the strong bonds of nitrogen gas molecules, allowing them to combine with hydrogen to form ammonia (NH3).The chemical equation for the Haber-Bosch process is:
This equation shows that one molecule of nitrogen gas reacts with three molecules of hydrogen gas to produce two molecules of ammonia gas. The process is very important for producing ammonia on a large scale, which is then used to make fertilizers, explosives, and other chemicals. The Haber-Bosch process is efficient and is the primary method for ammonia production worldwide.
| Property | Detail |
|---|---|
| Molecular Formula | NH₃ |
| Molecular Weight | 17.031 g/mol |
| State at Room Temperature | Gas |
| Color | Colorless |
| Odor | Sharp, pungent |
| Boiling Point | -33.34°C (-28.01°F) |
| Melting Point | -77.73°C (-107.91°F) |
| Solubility in Water | Highly soluble, forming a basic solution (ammonium hydroxide) |
| Density | 0.73 kg/m³ (gas at 0°C and 1 atm) |
| pH | About 11 when dissolved in water to form ammonium hydroxide |
Ammonia acts as a base when dissolved in water, producing hydroxide ions (OH-). This makes the solution alkaline.
Equation: NH₃ + H₂O → NH₄⁺ + OH⁻
Ammonia reacts with acids to form ammonium salts. For example, when it reacts with hydrochloric acid (HCl), ammonium chloride (NH4Cl) is formed.
Equation: NH₃ + HCl → NH₄Cl
Ammonia can burn in the presence of oxygen, but it requires a high temperature to ignite. The reaction with oxygen produces nitrogen gas (N2) and water (H2O).
Equation: 4NH₃ + 3O₂ → 2N₂ + 6H₂O
Ammonia can form complex compou
nds with metals. For instance, it forms a complex with copper sulfate, resulting in a deep blue solution. This is due to the formation of the [Cu(NH₃)₄]²⁺complex ion.
When heated, ammonia can decompose into its elements, nitrogen, and hydrogen. However, this requires very high temperatures and is not a common reaction under normal conditions.
| Property | Value |
|---|---|
| CAS registry number | 7664-41-7 |
| Beilstein number | 3587154 |
| PubChem compound ID | 222 |
| PubChem substance ID | 24857750 |
| SMILES identifier | N |
| InChI identifier | InChI=1/H3N/h1H3 |
| RTECS number | BO0875000 |
| MDL number | MFCD00011418 |
| Property | Value |
|---|---|
| NFPA health rating | 3 |
| NFPA fire rating | 1 |
| NFPA reactivity rating | 0 |
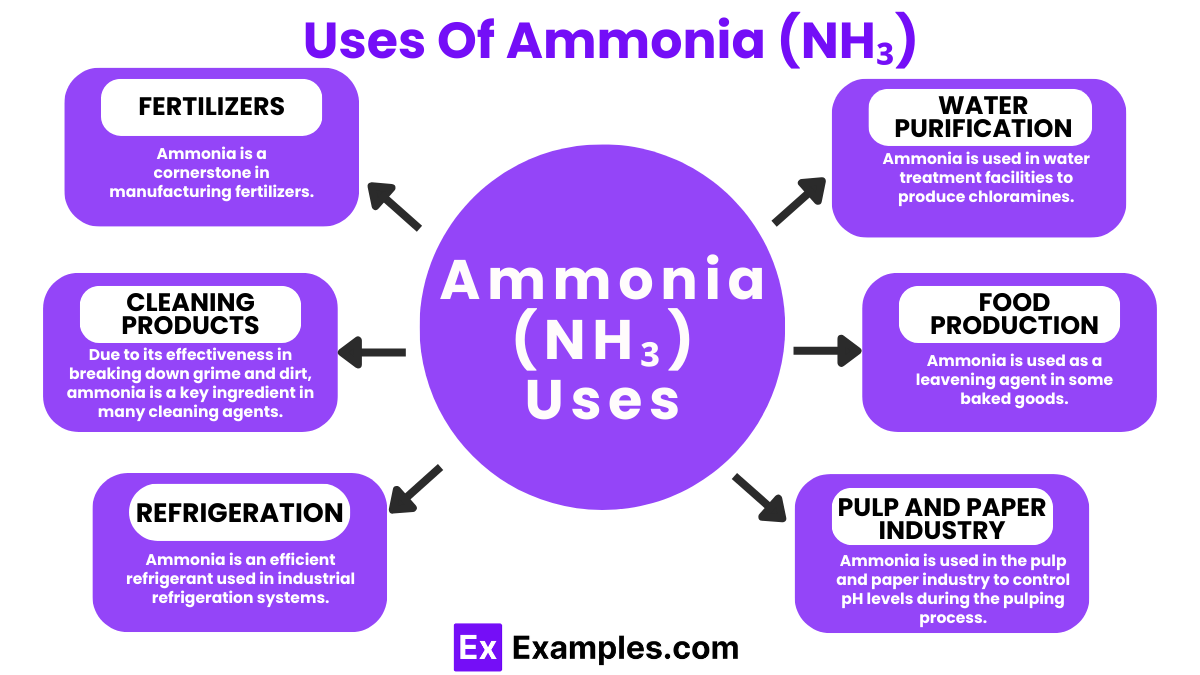
Ammonia is a cornerstone in manufacturing fertilizers. It’s directly applied to soil in its anhydrous form or used to produce nitrogen-rich compounds like urea, ammonium nitrate, and ammonium sulfate. These fertilizers are essential for promoting plant growth and increasing crop yields.
Due to its effectiveness in breaking down grime and dirt, ammonia is a key ingredient in many cleaning agents. It’s especially popular in solutions for cleaning windows and stainless steel, leaving surfaces sparkling without streaks.
Ammonia is an efficient refrigerant used in industrial refrigeration systems, including those in food processing plants and cold storage facilities. Its high energy efficiency and low environmental impact make it a preferred choice over some synthetic refrigerants.
Ammonia plays a role in producing various pharmaceutical products. It’s involved in synthesizing medications, such as antibiotics and vitamins, contributing to health and wellness.
In the textile industry, ammonia is used in pre-treatment processes of cotton materials. It helps in mercerization, where cotton fibers are treated with ammonia to increase their luster, strength, and affinity for dyes.
Ammonia is used in the pulp and paper industry to control pH levels during the pulping process. Adjusting the pH is crucial for improving the quality and durability of the paper.
Ammonia is used in water treatment facilities to produce chloramines. Chloramines are disinfectants used to purify drinking water, effectively killing harmful microorganisms while minimizing the formation of harmful by-products.
Ammonium nitrate, made from ammonia, is a key component in making explosives. These explosives are widely used in mining, construction, and demolition activities.
Ammonia is involved in producing plastics and synthetic fibers. It acts as a feedstock for producing acrylonitrile, which is a precursor for acrylic fibers and various plastics.
Ammonia is used as a leavening agent in some baked goods. It helps to adjust acidity levels in food, ensuring proper growth of yeasts and other microorganisms during fermentation.
Yes, ammonia can be toxic when inhaled, ingested, or if it comes into contact with skin, causing respiratory issues, skin burns, and eye irritation.
Both ammonia and vinegar are effective cleaners, but ammonia is stronger for tough grime, while vinegar is safer and eco-friendly for everyday use.
Avoid using ammonia on brass, copper, marble, and certain plastics as it can cause damage. Also, never mix ammonia with bleach due to toxic fumes.
Ammonia is produced naturally from the decomposition of organic matter and is also synthetically manufactured for industrial and agricultural uses.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the molecular formula of ammonia?
NH₂
NH₃
N2H4
NH₄
What is the shape of an ammonia molecule?
Linear
Trigonal planar
Tetrahedral
Trigonal pyramidal
Which type of bond is present between nitrogen and hydrogen in ammonia?
Ionic bond
Covalent bond
Metallic bond
Hydrogen bond
What is the primary use of ammonia in agriculture?
Pesticide
Herbicide
Fertilizer
Insecticide
What is the boiling point of ammonia at standard atmospheric pressure?
-33°C
0°C
25°C
100°C
Which process is used for the industrial synthesis of ammonia?
Haber process
Contact proce
Solvay process
Ostwald process
Ammonia can act as which type of agent in chemical reactions?
Oxidizing agent
Reducing agent
Neutral agent
Catalytic agent
What is the pH of a concentrated ammonia solution?
Acidic
Neutral
Basic
Amphoteric
Which of the following is a property of ammonia?
Colorless gas
Sweet odor
Heavy gas
Insoluble in water
Ammonia is used as a refrigerant because of its:
High boiling point
Low toxicity
High heat of vaporization
High flammability
Before you leave, take our quick quiz to enhance your learning!

