What is the most common form of nitrogen found in the atmosphere?
Nitrate
Nitrite
Ammonia
Nitrogen gas
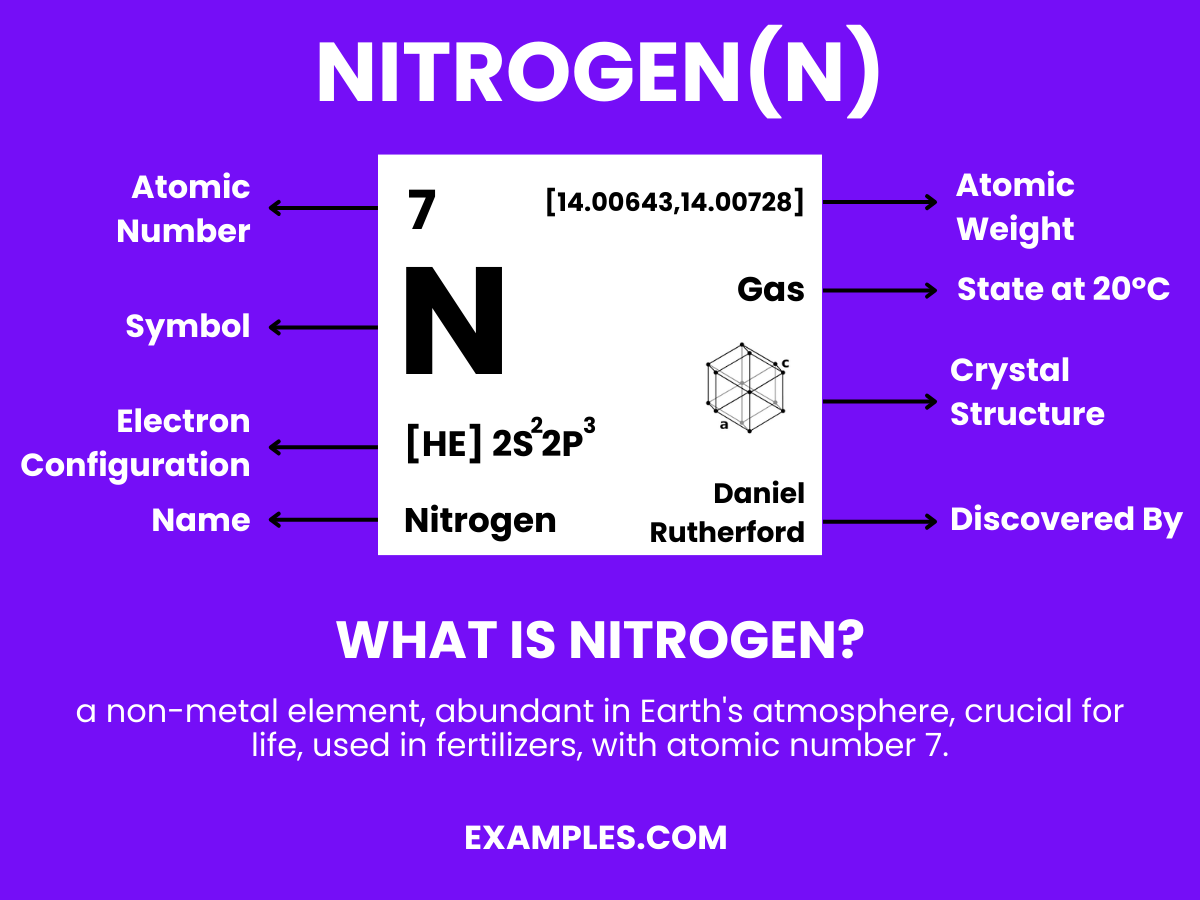
Discover Nitrogen, a fundamental element that pairs with hydrogen to form the building blocks of life. This guide takes you through the journey of Nitrogen’s role in the atmosphere, its usage in various industries, and its presence in organic compounds. Understanding Nitrogen is key in fields ranging from agriculture to medicine. Dive into real-world examples, explore its dynamic behaviors, and grasp its essentiality in forming amino acids, DNA, and the air we breathe.
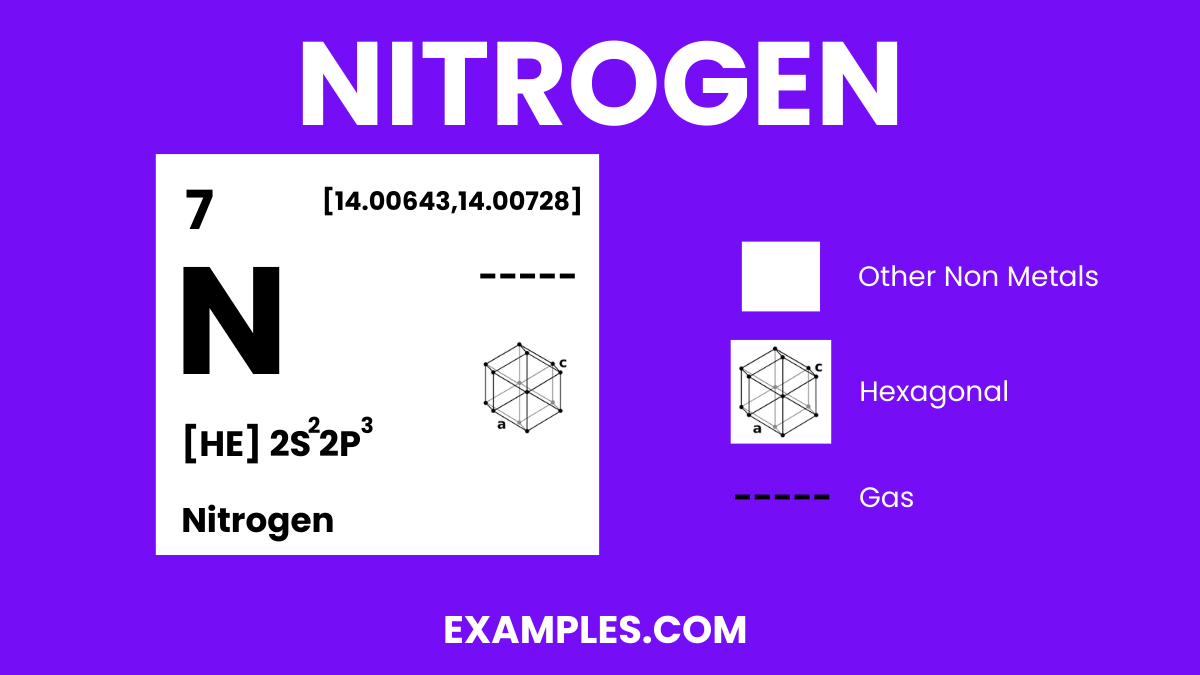
Nitrogen is a colorless, odorless, tasteless gas that constitutes about 78% of the Earth’s atmosphere by volume. Represented by the symbol ‘N’ and atomic number 7, it is a nonmetal with five electrons in its outer shell. Nitrogen is crucial for all living organisms as it is a key component of amino acids, proteins, and nucleic acids (DNA & RNA). It exists primarily as diatomic molecules (N₂), making it inert and stable under standard conditions. In the vast realm of chemistry, Nitrogen plays a pivotal role in creating a diverse range of compounds, from the simplicity of ammonia (NH₃) to the complexity of organic molecules, and is essential in both the industrial and biological spheres.
| Hydrogen | Sulfur |
| Carbon | Chlorine |
| Oxygen | Selenium |
| Fluorine | Bromine |
| Phosphorus | Iodine |
| Property | Description |
|---|---|
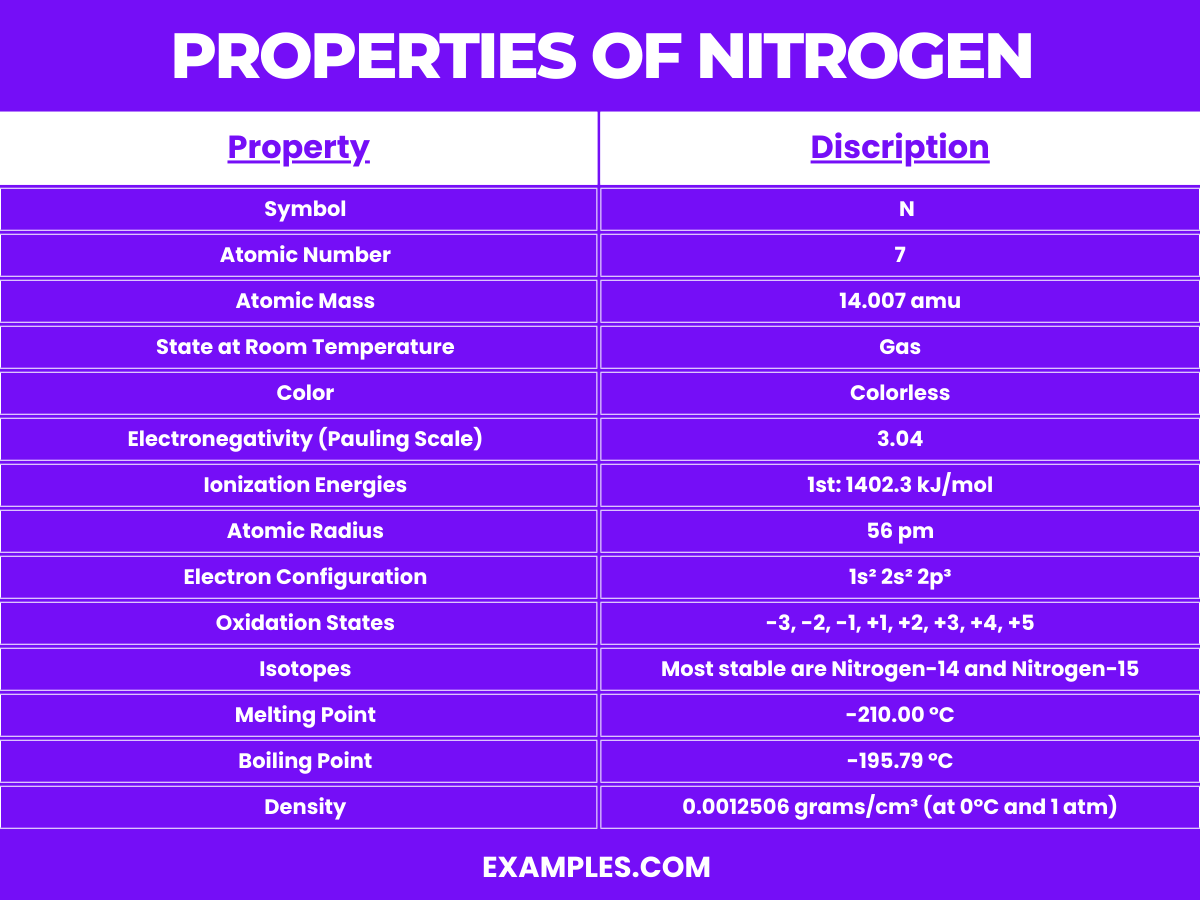
| Symbol | N |
| Atomic Number | 7 |
| Atomic Mass | 14.007 amu |
| State at Room Temperature | Gas |
| Color | Colorless |
| Electronegativity (Pauling Scale) | 3.04 |
| Ionization Energies | 1st: 1402.3 kJ/mol |
| Atomic Radius | 56 pm |
| Electron Configuration | 1s² 2s² 2p³ |
| Oxidation States | -3, -2, -1, +1, +2, +3, +4, +5 |
| Isotopes | Most stable are Nitrogen-14 and Nitrogen-15 |
| Reactivity | Generally inert due to strong triple bond in N₂, reactive under certain conditions |
| Compounds | Forms a variety of compounds like ammonia (NH₃), nitric acid (HNO₃), nitrates (NO₃⁻), and nitrites (NO₂⁻) |
| Melting Point | -210.00 °C |
| Boiling Point | -195.79 °C |
| Density | 0.0012506 grams/cm³ (at 0°C and 1 atm) |
| Solubility in Water | Poor, but soluble under high pressure and low temperature |
| Property | Value with Unit |
|---|---|
| Boiling Point | -195.79 °C |
| Melting Point | -210.00 °C |
| Critical Temperature | -147.1 °C |
| Critical Pressure | 3.39 MPa |
| Heat of Vaporization | 5.56 kJ/mol |
| Heat of Fusion | 0.72 kJ/mol |
| Specific Heat Capacity (at 15°C) | 1.04 J/g·K |
| Thermal Conductivity | 0.02583 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 0°C and 1 atm) | 1.2506 kg/m³ |
| Viscosity (at -195.79°C) | 0.000158 Pa·s |
| Solubility in Water (at 0°C) | 0.0235 g/100 mL of water |
| Refractive Index (at -183°C) | 1.000297 |
| Surface Tension (at -195.79°C) | 8.85 mN/m |
| Property | Value with Unit |
|---|---|
| Electric Dipole Moment | 0 Debye |
| Ionization Energy | 14.534 eV |
| Electron Affinity | -6.8 kJ/mol |
| Electrical Conductivity | Non-conductive |
| Magnetic Susceptibility | -12.0 × 10^-6 cm^3/mol |
| Property | Value with Unit |
|---|---|
| Atomic Number | 7 |
| Atomic Mass | 14.007 amu |
| Isotopes | ¹⁴N (99.63%), ¹⁵N (0.37%) |
| Nuclear Spin (for ¹⁴N) | 1 ℏ |
| Nuclear Spin (for ¹⁵N) | 1/2 ℏ |
| Neutron Cross Section (for ¹⁴N) | 1.83 barns |
| Neutron Cross Section (for ¹⁵N) | 0.000024 barns |
| Nuclear Magnetic Moment (for ¹⁴N) | 0.40376100 µN |
| Nuclear Magnetic Moment (for ¹⁵N) | -0.28318884 µN |
The Nitrogen Cycle is a biogeochemical process that illustrates the movement and transformation of nitrogen through various phases in the environment, including the atmosphere, lithosphere, hydrosphere, and biosphere. Here’s a simplified breakdown of the nitrogen cycle stages:
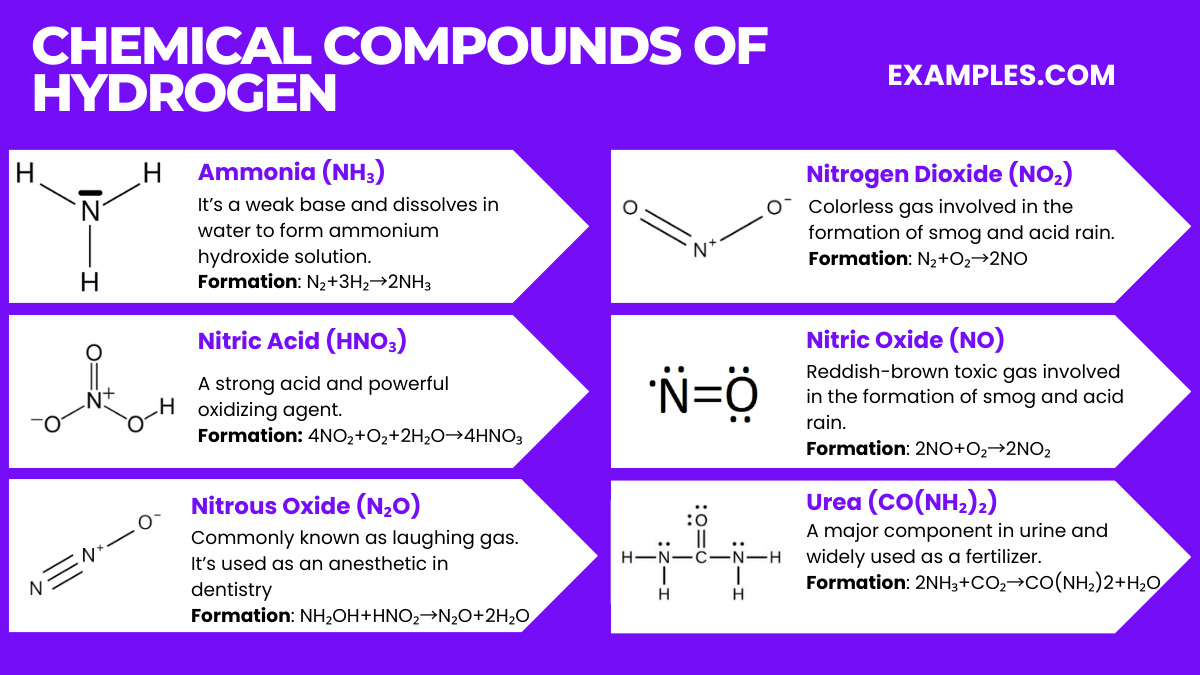
Nitrogen, a versatile and widely abundant element, forms a variety of chemical compounds that are essential to life and industry. Understanding these compounds provides insight into environmental processes, industrial applications, and biological functions. Here are some key nitrogen compounds:
| Isotope | Symbol | Neutrons | Protons | Natural Abundance | Properties |
|---|---|---|---|---|---|
| Nitrogen-14 | ¹⁴N | 7 | 7 | Approx. 99.63% | Stable, non-radioactive, most common isotope |
| Nitrogen-15 | ¹⁵N | 8 | 7 | Approx. 0.37% | Stable, non-radioactive, used as a tracer in scientific research |
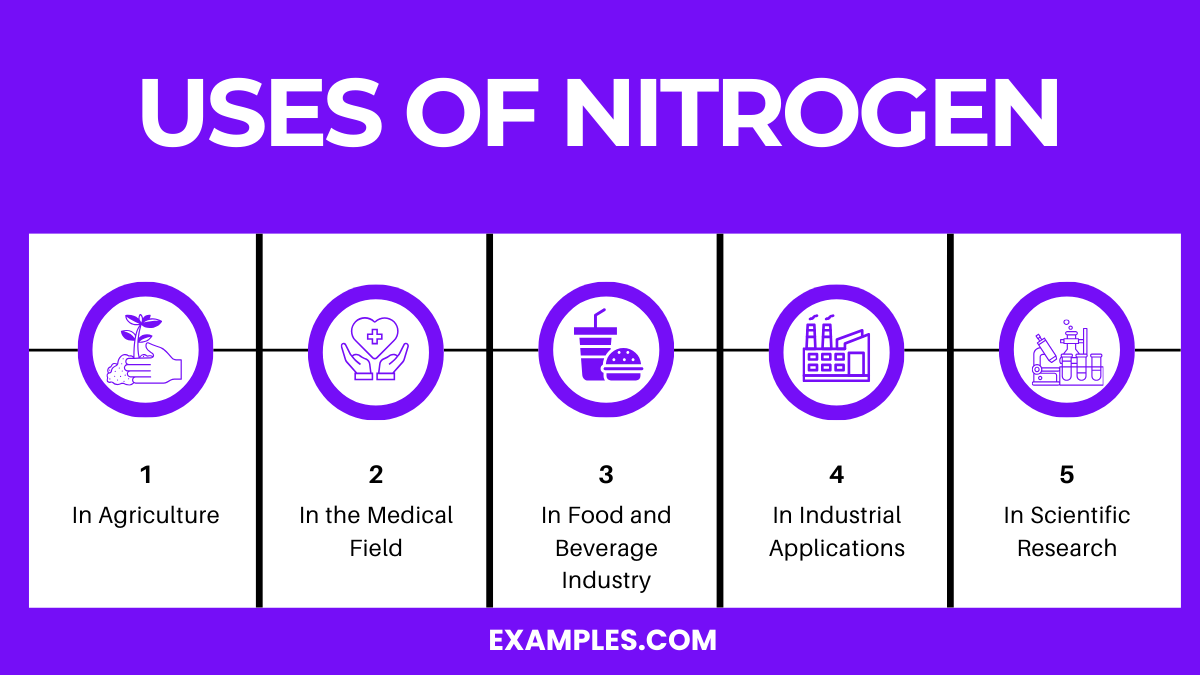
Nitrogen, a versatile and widely abundant element, is crucial in various sectors due to its unique properties. It’s the most abundant gas in Earth’s atmosphere and has numerous applications in the industrial, technological, and scientific sectors. Understanding its uses is pivotal for educators and students alike to appreciate this element’s versatility.
Nitrogen is one of the most abundantly available elements on earth, making up about 78% of the Earth’s atmosphere. Its vast availability makes it essential for various commercial and industrial applications. Here is a comprehensive guide to the commercial production of nitrogen, aimed at providing educators with detailed insights into the process.
Educators can utilize this comprehensive guide to enlighten students about the critical role nitrogen plays in everyday life, from the food we eat to the technologies we depend on. Understanding nitrogen’s uses not only conveys its importance in various industries but also instills a deeper appreciation for this fundamental element in the natural world.
Nitrogen, as an elemental gas in its diatomic form (N₂), is relatively inert and non-toxic, making up about 78% of the Earth’s atmosphere. However, its various compounds and forms can have different health effects, ranging from beneficial to harmful. Here are some of the health effects associated with nitrogen and its compounds:
Despite some of the hazards associated with nitrogen compounds, nitrogen also has numerous benefits and is essential for life:
Proteins, DNA, and chlorophyll are made up of nitrogen, essential for all living organisms’ structure and metabolic processes.
Nitrogen is used in fertilizers, food preservation, industrial applications, and medical procedures such as cryopreservation.
In humans, nitrogen is crucial for building proteins, nucleic acids, and is a significant component of our genetic material.
Nitrogen is a gas, making up about 78% of Earth’s atmosphere by volume; it’s colorless, odorless, and mostly inert.
Nitrogen is essential and non-toxic in its molecular form (N₂), but certain nitrogen compounds or excessive amounts can be harmful.
Nitrogen is a fundamental element that permeates life, industry, and the environment. Understanding its versatile roles, from its essential presence in organic compounds to its vast industrial applications, can enhance both educational and practical approaches to science. With this guide, embrace nitrogen’s complexities and its vital contributions to our world. Use this knowledge responsibly and innovatively for a sustainable future.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the most common form of nitrogen found in the atmosphere?
Nitrate
Nitrite
Ammonia
Nitrogen gas
Which of the following is a use of nitrogen in the industrial sector?
Ripening fruits
Welding and metal cutting
Water purification
Bleaching fabrics
What role does nitrogen play in the ecosystem?
Primary energy source
Helps in soil erosion
Nutrient for plant growth
Regulates water cycles
How do animals and humans primarily obtain nitrogen?
Breathing nitrogen gas
Consuming plants or plant-consuming animals
Drinking water
Exposure to sunlight
Which process converts atmospheric nitrogen into a usable form for plants?
Photosynthesis
Nitrification
Nitrogen fixation
Decomposition
Which compound results from the industrial fixation of nitrogen?
Nitric acid
Ammonia
Nitrogen gas
Nitrous oxide
What is a common agricultural problem associated with excess nitrogen?
Reduced crop yield
Soil acidification
Increased pest resistance
Enhanced flavor in crops
In which form is nitrogen most commonly absorbed by plants?
Nitrogen gas
Ammonium
Nitrate
Urea
What is the main environmental concern related to nitrogen-based fertilizers?
Air pollution
Water pollution
Noise pollution
Light pollution
Which enzyme is crucial for the process of nitrogen fixation in plants?
Amylase
Nitrogenase
Catalase
Rubisco
Before you leave, take our quick quiz to enhance your learning!

